The fracture mechanics of human bone: influence of disease and treatment
- PMID: 26380080
- PMCID: PMC4562496
- DOI: 10.1038/bonekey.2015.112
The fracture mechanics of human bone: influence of disease and treatment
Abstract
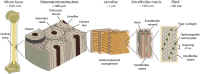
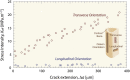
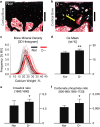
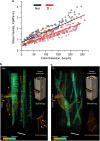
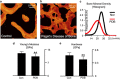
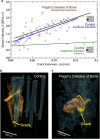
Aging and bone diseases are associated with increased fracture risk. It is therefore pertinent to seek an understanding of the origins of such disease-related deterioration in bone's mechanical properties. The mechanical integrity of bone derives from its hierarchical structure, which in healthy tissue is able to resist complex physiological loading patterns and tolerate damage. Indeed, the mechanisms through which bone derives its mechanical properties make fracture mechanics an ideal framework to study bone's mechanical resistance, where crack-growth resistance curves give a measure of the intrinsic resistance to the initiation of cracks and the extrinsic resistance to the growth of cracks. Recent research on healthy cortical bone has demonstrated how this hierarchical structure can develop intrinsic toughness at the collagen fibril scale mainly through sliding and sacrificial bonding mechanisms that promote plasticity. Furthermore, the bone-matrix structure develops extrinsic toughness at much larger micrometer length-scales, where the structural features are large enough to resist crack growth through crack-tip shielding mechanisms. Although healthy bone tissue can generally resist physiological loading environments, certain conditions such as aging and disease can significantly increase fracture risk. In simple terms, the reduced mechanical integrity originates from alterations to the hierarchical structure. Here, we review how human cortical bone resists fracture in healthy bone and how changes to the bone structure due to aging, osteoporosis, vitamin D deficiency and Paget's disease can affect the mechanical integrity of bone tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Weiner S, Wagner HD. The material bone: structure mechanical function relations. Annu Rev Mater Sci 1998; 28: 271–298.
-
- Arsenault A. Image-analysis of collagen-associated mineral distribution in cryogenically prepared turkey leg tendons. Calcif Tissue Int 1991; 48: 56–62. - PubMed
-
- Robins SP. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans 2007; 35: 849–852. - PubMed
-
- Thurner PJ, Katsamenis OL. The role of nanoscale toughening mechanisms in osteoporosis. Curr Osteoporos Rep 2014; 12: 351–356. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
