Organ Impairment-Drug-Drug Interaction Database: A Tool for Evaluating the Impact of Renal or Hepatic Impairment and Pharmacologic Inhibition on the Systemic Exposure of Drugs
- PMID: 26380158
- PMCID: PMC4562165
- DOI: 10.1002/psp4.55
Organ Impairment-Drug-Drug Interaction Database: A Tool for Evaluating the Impact of Renal or Hepatic Impairment and Pharmacologic Inhibition on the Systemic Exposure of Drugs
Abstract
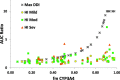
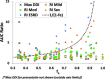
The organ impairment and drug-drug interaction (OI-DDI) database is the first rigorously assembled database of pharmacokinetic drug exposure data from publicly available renal and hepatic impairment studies presented together with the maximum change in drug exposure from drug interaction inhibition studies. The database was used to conduct a systematic comparison of the effect of renal/hepatic impairment and pharmacologic inhibition on drug exposure. Additional applications are feasible with the public availability of this database.
Figures

References
-
- Guidance for Industry: Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design, Data Analysis, and Impact on Dosing and Labeling, U.S. Food and Drug Administration. < http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... > (2003). Accessed January 21, 2015.
-
- Draft Guidance for Industry Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling, U.S. Food and Drug Adminstration. < http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati... > (2010). Accessed January 21, 2015.
-
- Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function, European Medicines Agency. < http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... > (2014). Accessed January 21, 2015.
-
- Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Impaired Hepatic Function, European Medicine Agency. < http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... > (2005). Accessed January 21, 2015.
-
- Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
LinkOut - more resources
Full Text Sources
Other Literature Sources

