The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma
- PMID: 26380916
- PMCID: PMC5215625
- DOI: 10.1002/jbm.b.33525
The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma
Abstract
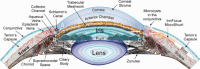
Glaucoma is the second leading cause of blindness with ∼70 million people worldwide who are blind from this disease. The currently practiced trabeculectomy surgery, the gold standard treatment used to stop the progression of vision loss, is rather draconian, traumatic to the patient and requires much surgical skill to perform. This article summarizes the more than 10-year development path of a novel device called the InnFocus MicroShunt®, which is a minimally invasive glaucoma drainage micro-tube used to shunt aqueous humor from the anterior chamber of the eye to a flap formed under the conjunctiva and Tenon's Capsule. The safety and clinical performance of this device approaches that of trabeculectomy. The impetus to develop this device stemmed from the invention of a new biomaterial called poly(styrene-block-isobutylene-block-styrene), or "SIBS." SIBS is ultra-stable with virtually no foreign body reaction in the body, which manifests in the eye as clinically insignificant inflammation and capsule formation. The quest for an easier, safer, and more effective method of treating glaucoma led to the marriage of SIBS with this glaucoma drainage micro-tube. This article summarizes the development of SIBS and the subsequent three iterations of design and four clinical trials that drove the one-year qualified success rate of the device from 43% to 100%. © 2015 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 105B: 211-221, 2017.
Keywords: SIBS; biodegradation; entrepreneurship; glaucoma; trabeculectomy.
© 2015 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc.
Figures







References
-
- Pinchuk L. A review of the biostability and carcinogenicity of polyurethanes in medicine and the new generation of ‘biostable’ polyurethanes. J Biomater Sci Polym Edn 1994;6:225–267. - PubMed
-
- Lamba NM, Woodhouse KA, Cooper SL. Polyurethanes in Biomedical Applications. CRC press; 1997.
-
- Vondráček P, Doležel B, Biostability of medical elastomers: A review. Biomaterials 1984;5:209–214. - PubMed
-
- Wolf CJ, Jerina KL, Brandon HJ, Young VL. The transport of octamethylcyclotetrasiloxane (D4) and polydimethylsiloxane (PDMS) in lightly cross‐linked silicone rubber. J Biomater Sci Polym Edn 2001;12:801–815. - PubMed
-
- Portney GL. Silicone elastomer implantation cyclodialysis. A negative report. Arch Ophthalmol 1973;89:10–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

