mTOR Overactivation and Compromised Autophagy in the Pathogenesis of Pulmonary Fibrosis
- PMID: 26382847
- PMCID: PMC4575195
- DOI: 10.1371/journal.pone.0138625
mTOR Overactivation and Compromised Autophagy in the Pathogenesis of Pulmonary Fibrosis
Abstract
The mammalian target of rapamycin (mTOR) signaling pathway in pulmonary fibrosis was investigated in cell and animal models. mTOR overactivation in alveolar epithelial cells (AECs) was achieved in the conditional and inducible Tsc1 knock-down mice SPC-rtTA/TetO-Cre/Tsc1(fx/+) (STT). Doxycycline caused Tsc1 knock-down and consequently mTOR activation in AECs for the STT mice. Mice treated with bleomycin exhibited increased mortality and pulmonary fibrosis compared with control mice. In wild-type C57BL/6J mice, pretreatment with rapamycin attenuated the bleomycin-mediated mortality and fibrosis. Rapamycin-mediated mouse survival benefit was inhibited by chloroquine, an autophagy inhibitor. Autophagosomes were decreased in the lungs after bleomycin exposure. Rapamycin induced the production of autophagosomes and diminished p62. We concluded that mTOR overactivation in AECs and compromised autophagy in the lungs are involved in the pathogenesis of pulmonary fibrosis. The suppression of mTOR and enhancement of autophagy may be used for treatment of pulmonary fibrosis.
Conflict of interest statement
Figures
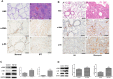


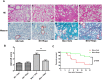
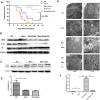
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183(6):788–824. Epub 2011/04/08. 10.1164/rccm.2009-040GL . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

