Integrated isotope-assisted metabolomics and (13)C metabolic flux analysis reveals metabolic flux redistribution for high glucoamylase production by Aspergillus niger
- PMID: 26383080
- PMCID: PMC4574132
- DOI: 10.1186/s12934-015-0329-y
Integrated isotope-assisted metabolomics and (13)C metabolic flux analysis reveals metabolic flux redistribution for high glucoamylase production by Aspergillus niger
Abstract
Background: Aspergillus niger is widely used for enzyme production and achievement of high enzyme production depends on the comprehensive understanding of cell's metabolic regulation mechanisms.
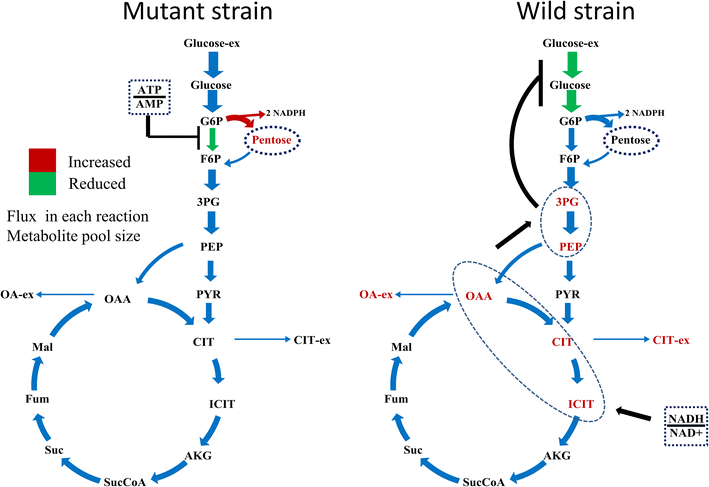
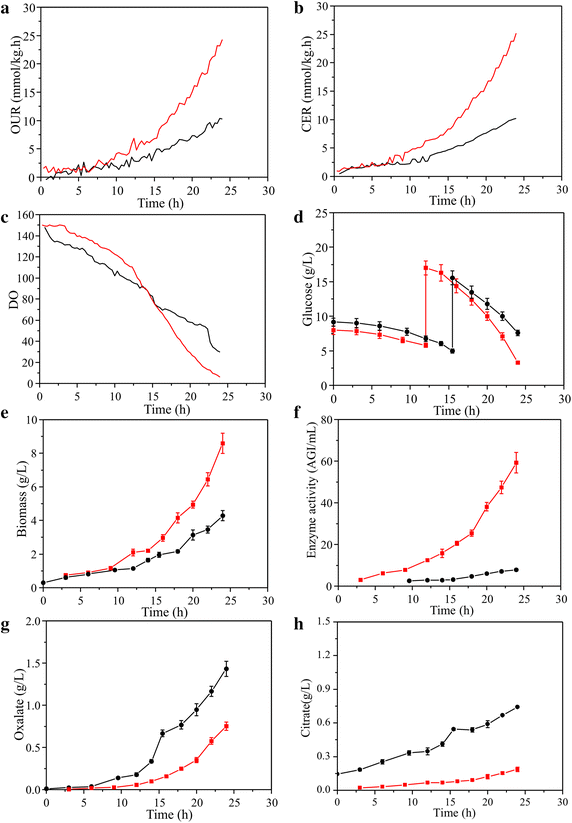
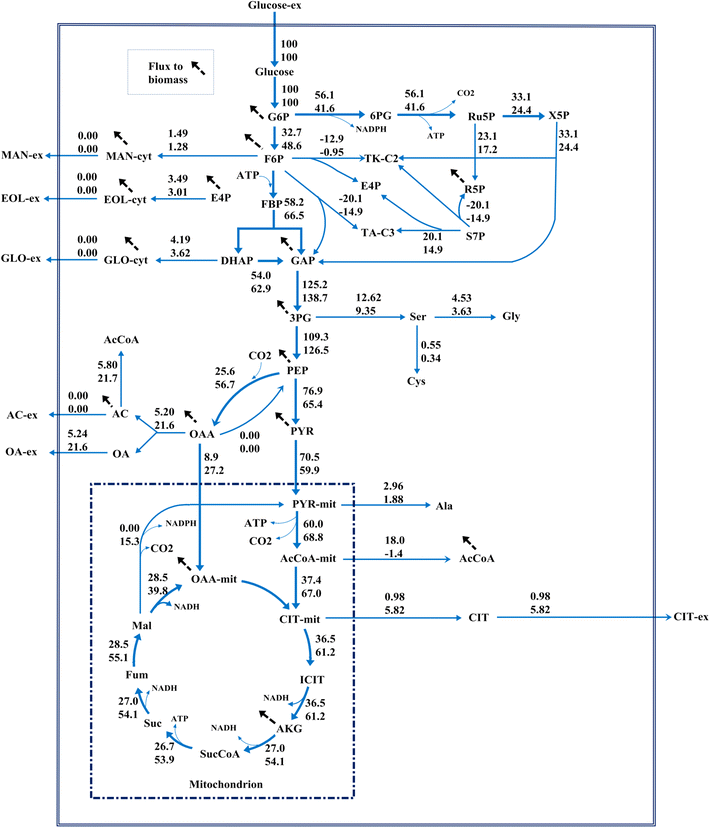
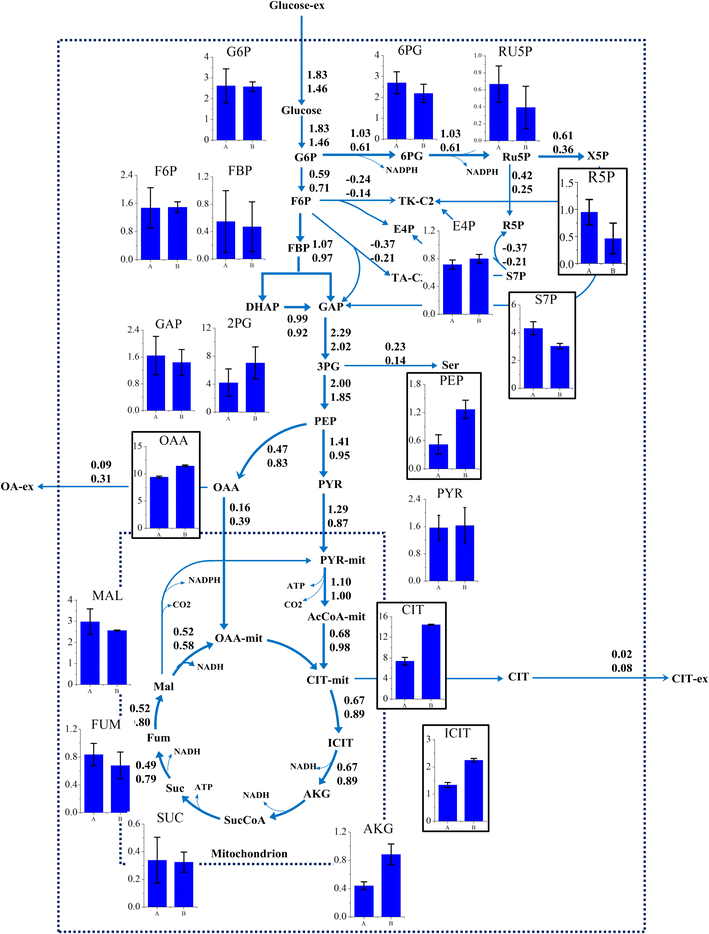
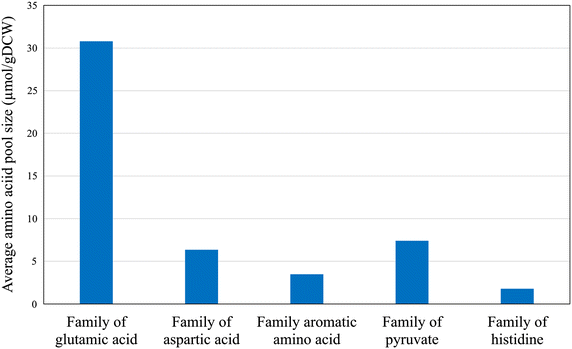
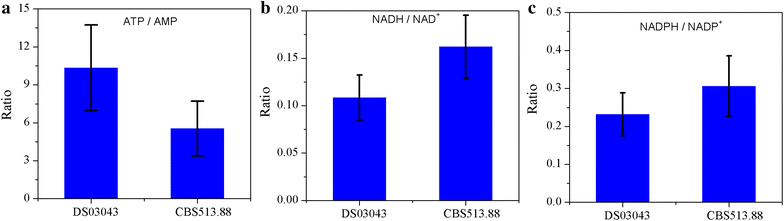
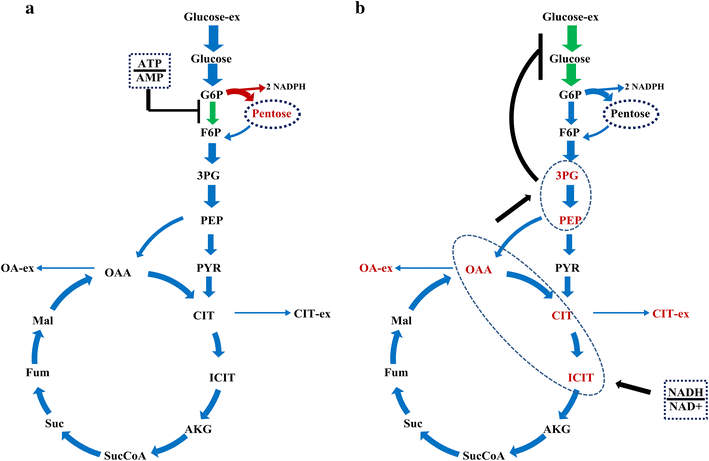
Results: In this paper, we investigate the metabolic differences and regulation mechanisms between a high glucoamylase-producing strain A. niger DS03043 and its wild-type parent strain A. niger CBS513.88 via an integrated isotope-assisted metabolomics and (13)C metabolic flux analysis approach. We found that A. niger DS03043 had higher cell growth, glucose uptake, and glucoamylase production rates but lower oxalic acid and citric acid secretion rates. In response to above phenotype changes, A. niger DS03043 was characterized by an increased carbon flux directed to the oxidative pentose phosphate pathway in contrast to reduced flux through TCA cycle, which were confirmed by consistent changes in pool sizes of metabolites. A higher ratio of ATP over AMP in the high producing strain might contribute to the increase in the PP pathway flux as glucosephosphate isomerase was inhibited at higher ATP concentrations. A. niger CBS513.88, however, was in a higher redox state due to the imbalance of NADH regeneration and consumption, resulting in the secretion of oxalic acid and citric acid, as well as the accumulation of intracellular OAA and PEP, which may in turn result in the decrease in the glucose uptake rate.
Conclusions: The application of integrated metabolomics and (13)C metabolic flux analysis highlights the regulation mechanisms of energy and redox metabolism on flux redistribution in A. niger. Graphical abstract An integrated isotope-assisted metabolomics and (13)C metabolic flux analysis was was firstly systematically performed in A. niger. In response to enzyme production, the metabolic flux in A. niger DS03043 (high-producing) was redistributed, characterized by an increased carbon flux directed to the oxidative pentose phosphate pathway as well as an increased pool size of pentose. The consistency in (13)C metabolic flux analysis and metabolites quantification indicated that an imbalance of NADH formation and consumption led to the accumulation and secretion of organic acids in A. niger CBS513.88 (wild-type).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

