Voltage-gated Ca(2+) influx through L-type channels contributes to sarcoplasmic reticulum Ca(2+) loading in skeletal muscle
- PMID: 26383921
- PMCID: PMC4626550
- DOI: 10.1113/JP270252
Voltage-gated Ca(2+) influx through L-type channels contributes to sarcoplasmic reticulum Ca(2+) loading in skeletal muscle
Abstract
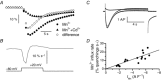
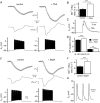
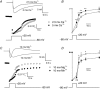
Muscle contraction is triggered by Ca(2+) ions released from the sarcoplasmic reticulum (SR) in response to depolarization of skeletal muscle fibres. Muscle activation is also associated with a voltage-activated trans-sarcolemmal Ca(2+) influx early identified as a current flowing through L-type Ca(2+) channels. Because removal of external Ca(2+) does not impede fibres from contracting, a negligible role was given to this voltage-activated Ca(2+) entry, although the decline of Ca(2+) release is more pronounced in the absence of Ca(2+) during long-lasting activation. Furthermore, it is not clearly established whether Ca(2+) exclusively flows through L-type channels or in addition through a parallel voltage-activated pathway distinct from L-type channels. Here, by monitoring the quenching of fura-2 fluorescence resulting from Mn(2+) influx in voltage-controlled mouse and zebrafish isolated muscle fibres, we show that the L-type current is the only contributor to Ca(2+) influx during long-lasting depolarizations in skeletal muscle. Calibration of the Mn(2+) quenching signal allowed us to estimate a mean Mn(2+) current of 0.31 ± 0.06 A F(-1) flowing through L-type channels during a train of action potentials. Measurements of SR Ca(2+) changes with fluo-5N in response to depolarization revealed that an elevated voltage-activated Ca(2+) current potentiated SR Ca(2+) loading and addition of external Mn(2+) produced quenching of fluo-5N in the SR, indicating that voltage-activated Ca(2+) /Mn(2+) influx contributes to SR Ca(2+) /Mn(2+) loading.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures







Similar articles
-
Preserved Ca2+ handling and excitation-contraction coupling in muscle fibres from diet-induced obese mice.Diabetologia. 2020 Nov;63(11):2471-2481. doi: 10.1007/s00125-020-05256-8. Epub 2020 Aug 25. Diabetologia. 2020. PMID: 32840676
-
Major contribution of sarcoplasmic reticulum Ca(2+) depletion during long-lasting activation of skeletal muscle.J Gen Physiol. 2013 May;141(5):557-65. doi: 10.1085/jgp.201310957. J Gen Physiol. 2013. PMID: 23630339 Free PMC article.
-
Coordinated control of cell Ca(2+) loading and triggered release from the sarcoplasmic reticulum underlies the rapid inotropic response to increased L-type Ca(2+) current.Circ Res. 2001 Feb 2;88(2):195-201. doi: 10.1161/01.res.88.2.195. Circ Res. 2001. PMID: 11157672
-
Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle.Cell Calcium. 2007 Oct-Nov;42(4-5):513-20. doi: 10.1016/j.ceca.2007.04.010. Epub 2007 Jun 7. Cell Calcium. 2007. PMID: 17559931 Review.
-
Molecular aspects of the excitation-contraction coupling in skeletal muscle.Jpn J Physiol. 1999 Aug;49(4):325-33. doi: 10.2170/jjphysiol.49.325. Jpn J Physiol. 1999. PMID: 10529492 Review.
Cited by
-
Porcine deltacoronavirus (PDCoV) modulates calcium influx to favor viral replication.Virology. 2020 Jan 2;539:38-48. doi: 10.1016/j.virol.2019.10.011. Epub 2019 Oct 22. Virology. 2020. PMID: 31670218 Free PMC article.
-
Superfast excitation-contraction coupling in adult zebrafish skeletal muscle fibers.J Gen Physiol. 2022 Sep 5;154(9):e202213158. doi: 10.1085/jgp.202213158. Epub 2022 Jun 29. J Gen Physiol. 2022. PMID: 35767225 Free PMC article.
-
Reduced voltage-activated Ca2+ release flux in muscle fibers from a rat model of Duchenne dystrophy.J Gen Physiol. 2025 Mar 3;157(2):e202413588. doi: 10.1085/jgp.202413588. Epub 2024 Dec 24. J Gen Physiol. 2025. PMID: 39718509
-
The voltage sensor of excitation-contraction coupling in mammals: Inactivation and interaction with Ca2.J Gen Physiol. 2017 Nov 6;149(11):1041-1058. doi: 10.1085/jgp.201611725. Epub 2017 Oct 11. J Gen Physiol. 2017. PMID: 29021148 Free PMC article.
-
Functional impact of an oculopharyngeal muscular dystrophy mutation in PABPN1.J Physiol. 2017 Jul 1;595(13):4167-4187. doi: 10.1113/JP273948. Epub 2017 Apr 25. J Physiol. 2017. PMID: 28303574 Free PMC article.
References
-
- Armstrong CM, Bezanilla FM & Horowicz P (1972). Twitches in the presence of ethylene glycol bis(‐aminoethyl ether)‐N,N′‐tetracetic acid. Biochim Biophys Acta 267, 605–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

