Rint1 inactivation triggers genomic instability, ER stress and autophagy inhibition in the brain
- PMID: 26383973
- PMCID: PMC5072440
- DOI: 10.1038/cdd.2015.113
Rint1 inactivation triggers genomic instability, ER stress and autophagy inhibition in the brain
Abstract
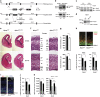
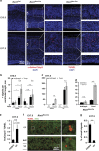
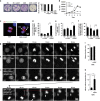
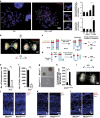
Endoplasmic reticulum (ER) stress, defective autophagy and genomic instability in the central nervous system are often associated with severe developmental defects and neurodegeneration. Here, we reveal the role played by Rint1 in these different biological pathways to ensure normal development of the central nervous system and to prevent neurodegeneration. We found that inactivation of Rint1 in neuroprogenitors led to death at birth. Depletion of Rint1 caused genomic instability due to chromosome fusion in dividing cells. Furthermore, Rint1 deletion in developing brain promotes the disruption of ER and Cis/Trans Golgi homeostasis in neurons, followed by ER-stress increase. Interestingly, Rint1 deficiency was also associated with the inhibition of the autophagosome clearance. Altogether, our findings highlight the crucial roles of Rint1 in vivo in genomic stability maintenance, as well as in prevention of ER stress and autophagy.
Figures
Similar articles
-
RINT1 Loss Impairs Retinogenesis Through TRP53-Mediated Apoptosis.Front Cell Dev Biol. 2020 Jul 30;8:711. doi: 10.3389/fcell.2020.00711. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850831 Free PMC article.
-
RINT1 functions as a multitasking protein at the crossroads between genomic stability, ER homeostasis, and autophagy.Autophagy. 2016 Aug 2;12(8):1413-5. doi: 10.1080/15548627.2016.1191730. Epub 2016 Jul 1. Autophagy. 2016. PMID: 27367497 Free PMC article.
-
Evaluation of Rint1 as a modifier of intestinal tumorigenesis and cancer risk.PLoS One. 2017 Mar 6;12(3):e0172247. doi: 10.1371/journal.pone.0172247. eCollection 2017. PLoS One. 2017. PMID: 28264000 Free PMC article.
-
The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family.Oncogene. 2008 Oct 27;27(50):6419-33. doi: 10.1038/onc.2008.309. Oncogene. 2008. PMID: 18955970 Review.
-
Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis.J Pineal Res. 2015 Oct;59(3):292-307. doi: 10.1111/jpi.12264. Epub 2015 Aug 9. J Pineal Res. 2015. PMID: 26201382 Review.
Cited by
-
Cell Type-Specific Role of RNA Nuclease SMG6 in Neurogenesis.Cells. 2021 Nov 30;10(12):3365. doi: 10.3390/cells10123365. Cells. 2021. PMID: 34943873 Free PMC article.
-
Disorders of vesicular trafficking presenting with recurrent acute liver failure: NBAS, RINT1, and SCYL1 deficiency.J Inherit Metab Dis. 2025 Jan;48(1):e12707. doi: 10.1002/jimd.12707. Epub 2024 Jan 27. J Inherit Metab Dis. 2025. PMID: 38279772 Free PMC article. Review.
-
An Expansion of the Endoplasmic Reticulum that Halts Autophagy is Permissive to Genome Instability.Contact (Thousand Oaks). 2023 Feb 22;6:25152564231157706. doi: 10.1177/25152564231157706. eCollection 2023 Jan-Dec. Contact (Thousand Oaks). 2023. PMID: 37366415 Free PMC article.
-
RINT1 Loss Impairs Retinogenesis Through TRP53-Mediated Apoptosis.Front Cell Dev Biol. 2020 Jul 30;8:711. doi: 10.3389/fcell.2020.00711. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850831 Free PMC article.
-
RINT1 deficiency disrupts lipid metabolism and underlies a complex hereditary spastic paraplegia.J Clin Invest. 2023 Jul 17;133(14):e162836. doi: 10.1172/JCI162836. J Clin Invest. 2023. PMID: 37463447 Free PMC article.
References
-
- Atkin JD, Farg MA, Soo KY, Walker AK, Halloran M, Turner BJ et al. Mutant SOD1 inhibits ER-Golgi transport in amyotrophic lateral sclerosis. J Neurochem 2014; 129: 190–204. - PubMed
-
- Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ 2009; 16: 984–990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

