Mosquito Rasputin interacts with chikungunya virus nsP3 and determines the infection rate in Aedes albopictus
- PMID: 26384002
- PMCID: PMC4573678
- DOI: 10.1186/s13071-015-1070-4
Mosquito Rasputin interacts with chikungunya virus nsP3 and determines the infection rate in Aedes albopictus
Abstract
Background: Chikungunya virus (CHIKV) is an arthritogenic alphavirus (family Togaviridae), transmitted by Aedes species mosquitoes. CHIKV re-emerged in 2004 with multiple outbreaks worldwide and recently reached the Americas where it has infected over a million individuals in a rapidly expanding epidemic. While alphavirus replication is well understood in general, the specific function (s) of non-structural protein nsP3 remain elusive. CHIKV nsP3 modulates the mammalian stress response by preventing stress granule formation through sequestration of G3BP. In mosquitoes, nsP3 is a determinant of vector specificity, but its functional interaction with mosquito proteins is unclear.
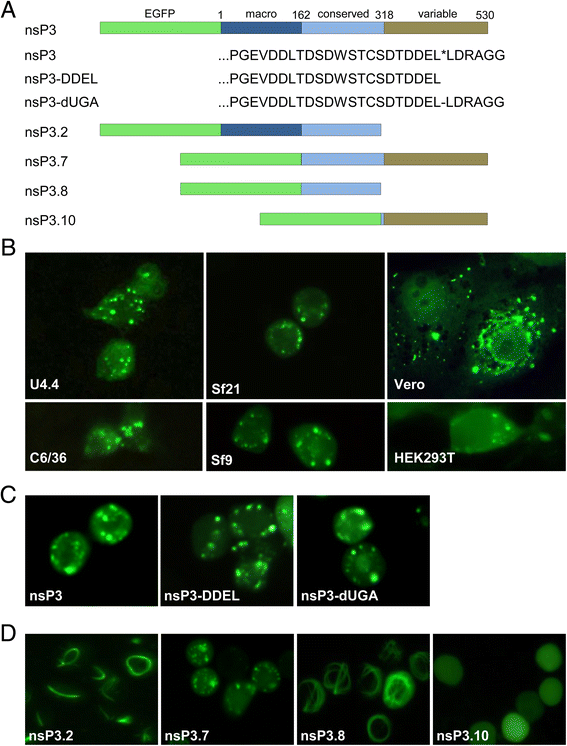
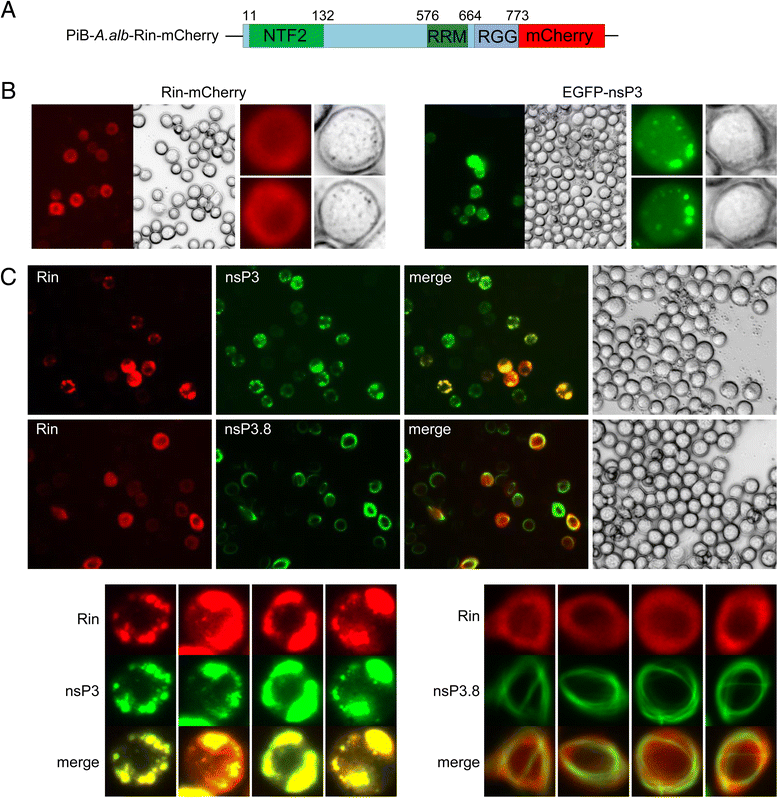
Methods: In this research we studied the domains required for localization of CHIKV nsP3 in insect cells and demonstrated its molecular interaction with Rasputin (Rin), the mosquito homologue of G3BP. The biological involvement of Rin in CHIKV infection was investigated in live Ae. albopictus mosquitoes.
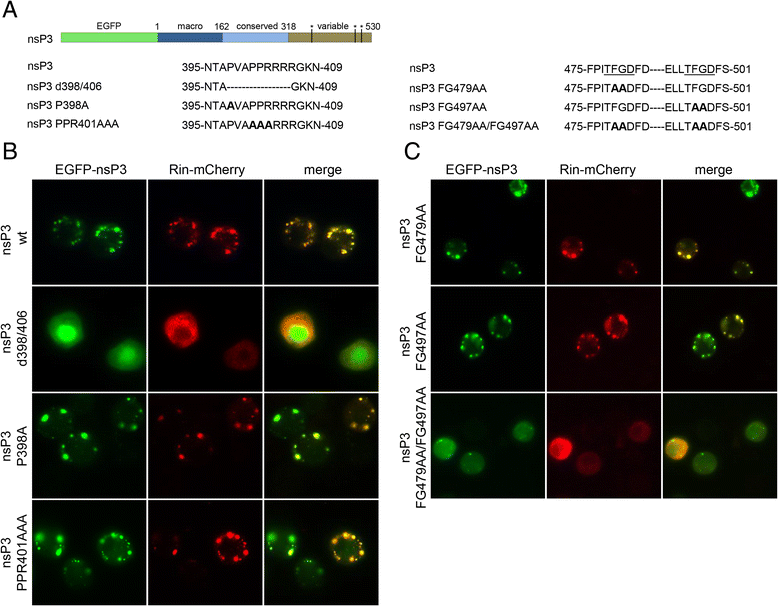
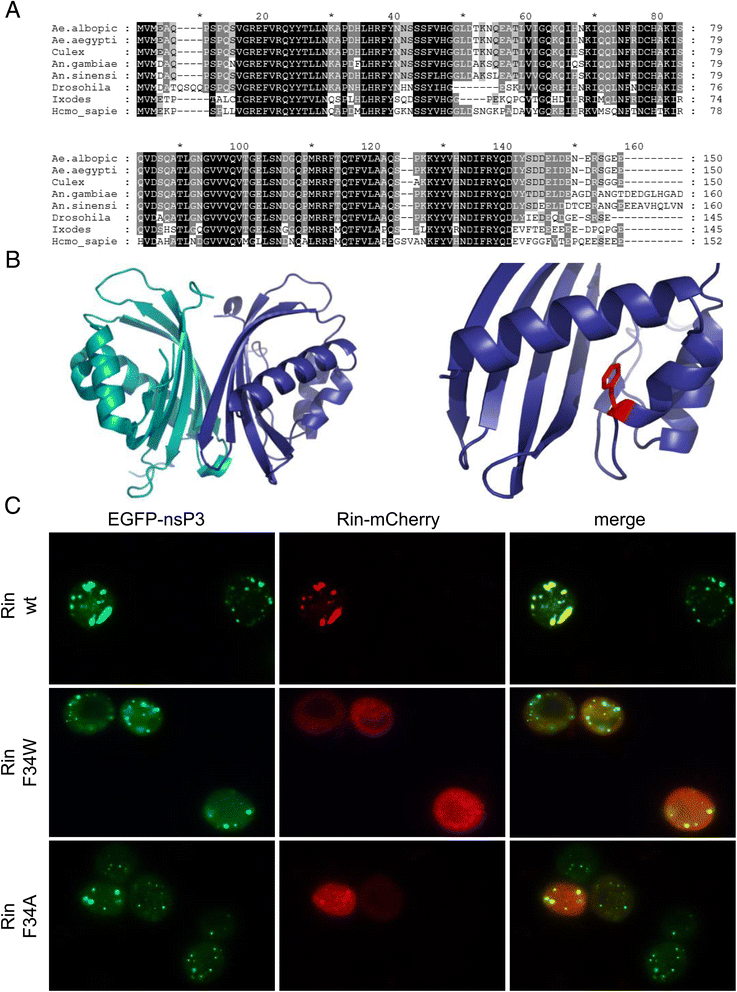
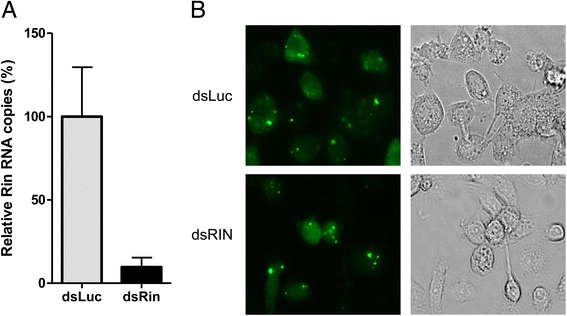
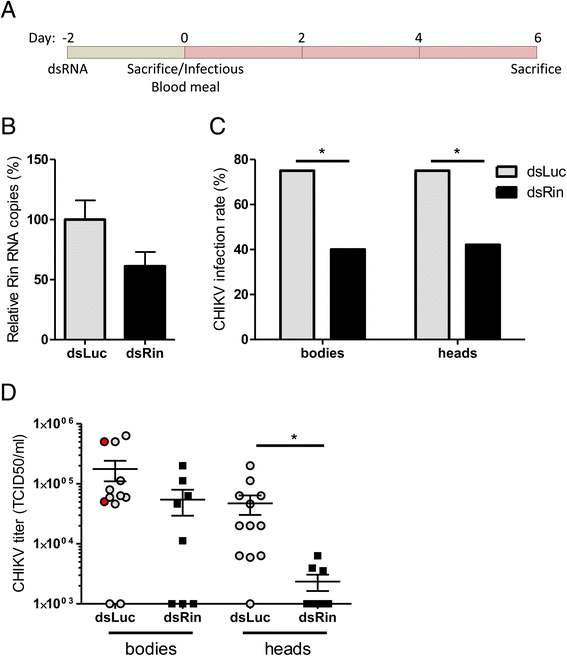
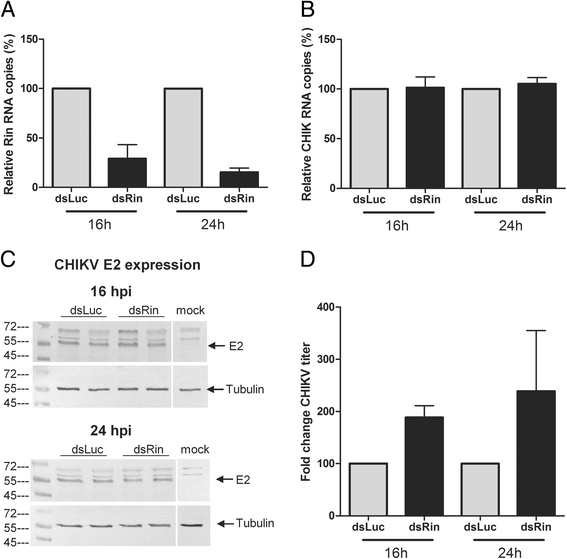
Results: In insect cells, nsP3 localized as cytoplasmic granules, which was dependent on the central domain and the C-terminal variable region but independent of the N-terminal macrodomain. Ae. albopictus Rin displayed a diffuse, cytoplasmic localization, but was effectively sequestered into nsP3-granules upon nsP3 co-expression. Site-directed mutagenesis showed that the Rin-nsP3 interaction involved the NTF2-like domain of Rin and two conserved TFGD repeats in the C-terminal variable domain of nsP3. Although in vitro silencing of Rin did not impact nsP3 localization or CHIKV replication in cell culture, Rin depletion in vivo significantly decreased the CHIKV infection rate and transmissibility in Ae.albopictus.
Conclusions: We identified the nsP3 hypervariable C-terminal domain as a critical factor for granular localization and sequestration of mosquito Rin. Our study offers novel insight into a conserved virus-mosquito interaction at the molecular level, and reveals a strong proviral role for G3BP homologue Rin in live mosquitoes, making the nsP3-Rin interaction a putative target to interfere with the CHIKV transmission cycle.
Figures
References
-
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007/12/07 ed. 2007;370: 1840–1846. doi:10.1016/S0140-6736(07)61779-6 - PubMed
-
- ECDC_Epidemiological update- autochthonous cases of chikungunya fever in France.pdf [Internet]. Available: www.ecdc.europa.eu.
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

