Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis
- PMID: 26384035
- PMCID: PMC9235409
- DOI: 10.1002/14651858.CD011381.pub2
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis
Update in
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article.
Abstract
Background: Different therapeutic strategies are available for the treatment of people with relapsing-remitting multiple sclerosis (RRMS), including immunomodulators, immunosuppressants and biologics. Although there is consensus that these therapies reduce the frequency of relapses, their relative benefit in delaying new relapses or disability worsening remains unclear due to the limited number of direct comparison trials.
Objectives: To compare the benefit and acceptability of interferon beta-1b, interferon beta-1a (Avonex, Rebif), glatiramer acetate, natalizumab, mitoxantrone, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab, pegylated interferon beta-1a, daclizumab, laquinimod, azathioprine and immunoglobulins for the treatment of people with RRMS and to provide a ranking of these treatments according to their benefit and acceptability, defined as the proportion of participants who withdrew due to any adverse event.
Search methods: We searched the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group Trials Register, which contains trials from CENTRAL (2014, Issue 9), MEDLINE (1966 to 2014), EMBASE (1974 to 2014), CINAHL (1981 to 2014), LILACS (1982 to 2014), clinicaltrials.gov and the WHO trials registry, and US Food and Drug Administration (FDA) reports. We ran the most recent search in September 2014.
Selection criteria: Randomised controlled trials (RCTs) that studied one or more of the 15 treatments as monotherapy, compared to placebo or to another active agent, for use in adults with RRMS.
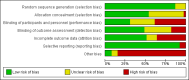
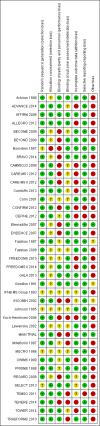
Data collection and analysis: Two authors independently identified studies from the search results and performed data extraction. We performed data synthesis by pairwise meta-analysis and network meta-analysis. We assessed the quality of the body of evidence for outcomes within the network meta-analysis according to GRADE, as very low, low, moderate or high.
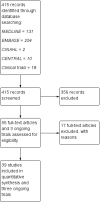
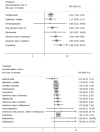
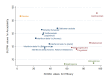
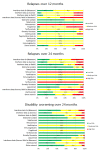
Main results: We included 39 studies in this review, in which 25,113 participants were randomised. The majority of the included trials were short-term studies, with a median duration of 24 months. Twenty-four (60%) were placebo-controlled and 15 (40%) were head-to-head studies.Network meta-analysis showed that, in terms of a protective effect against the recurrence of relapses in RRMS during the first 24 months of treatment, alemtuzumab, mitoxantrone, natalizumab, and fingolimod outperformed other drugs. The most effective drug was alemtuzumab (risk ratio (RR) versus placebo 0.46, 95% confidence interval (CI) 0.38 to 0.55; surface under the cumulative ranking curve (SUCRA) 96%; moderate quality evidence), followed by mitoxantrone (RR 0.47, 95% CI 0.27 to 0.81; SUCRA 92%; very low quality evidence), natalizumab (RR 0.56, 95% CI 0.47 to 0.66; SUCRA 88%; high quality evidence), and fingolimod (RR 0.72, 95% CI 0.64 to 0.81; SUCRA 71%; moderate quality evidence).Disability worsening was based on a surrogate marker, defined as irreversible worsening confirmed at three-month follow-up, measured during the first 24 months in the majority of included studies. Both direct and indirect comparisons revealed that the most effective treatments were mitoxantrone (RR versus placebo 0.20, 95% CI 0.05 to 0.84; SUCRA 96%; low quality evidence), alemtuzumab (RR 0.35, 95% CI 0.26 to 0.48; SUCRA 94%; low quality evidence), and natalizumab (RR 0.64, 95% CI 0.49 to 0.85; SUCRA 74%; moderate quality evidence).Almost all of the agents included in this review were associated with a higher proportion of participants who withdrew due to any adverse event compared to placebo. Based on the network meta-analysis methodology, the corresponding RR estimates versus placebo over the first 24 months of follow-up were: mitoxantrone 9.92 (95% CI 0.54 to 168.84), fingolimod 1.69 (95% CI 1.32 to 2.17), natalizumab 1.53 (95% CI 0.93 to 2.53), and alemtuzumab 0.72 (95% CI 0.32 to 1.61).Information on serious adverse events (SAEs) was scanty, characterised by heterogeneous results and based on a very low number of events observed during the short-term duration of the trials included in this review.
Authors' conclusions: Conservative interpretation of these results is warranted, since most of the included treatments have been evaluated in few trials. The GRADE approach recommends providing implications for practice based on moderate to high quality evidence. Our review shows that alemtuzumab, natalizumab, and fingolimod are the best choices for preventing clinical relapses in people with RRMS, but this evidence is limited to the first 24 months of follow-up. For the prevention of disability worsening in the short term (24 months), only natalizumab shows a beneficial effect on the basis of moderate quality evidence (all of the other estimates were based on low to very low quality evidence). Currently, therefore, insufficient evidence is available to evaluate treatments for the prevention of irreversible disability worsening.There are two additional major concerns that have to be considered. First, the benefit of all of these treatments beyond two years is uncertain and this is a relevant issue for a disease with a duration of 30 to 40 years. Second, short-term trials provide scanty and poorly reported safety data and do not provide useful evidence in order to obtain a reliable risk profile of treatments. In order to provide long-term information on the safety of the treatments included in this review, it will be necessary also to evaluate non-randomised studies and post-marketing reports released from the regulatory agencies. Finally, more than 70% of the studies included in this review were sponsored by pharmaceutical companies and this may have influenced the results.There are three needs that the research agenda should address. First, randomised trials of direct comparisons between active agents would be useful, avoiding further placebo-controlled studies. Second, follow-up of the original trial cohorts should be mandatory. Third, more studies are needed to assess the medium and long-term benefit and safety of immunotherapies and the comparative safety of different agents.
Conflict of interest statement
GF: none
GS: none
CDG: none
IT: none
RD: none
Figures

















References
References to studies included in this review
Achiron 1998 {published data only}
-
- Achiron A, Gabbay U, Gilad R, Hassin B, Barak Y, Gornish M, et al. Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology 1998;50(2):398‐402. - PubMed
ADVANCE 2014 {published data only}
-
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Pegylated interferon beta‐1a for relapsing‐remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double‐blind study. Lancet Neurology 2014;13:657‐65. - PubMed
AFFIRM 2006 {published data only}
-
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):899‐910. - PubMed
ALLEGRO 2012 {published data only}
-
- Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, et al. Placebo‐controlled trial of oral laquinimod for multiple sclerosis. New England Journal of Medicine 2012;366(11):1000‐9. - PubMed
BECOME 2009 {published data only}
-
- Cadavid D, Wolansky LJ, Skurnick J, Lincoln J, Cheriyan J, Szczepanowski K, et al. Efficacy of treatment of MS with IFNbeta‐1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72(23):1976‐83. - PubMed
BEYOND 2009 {published data only}
-
- O'Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, et al. 250 microg or 500 microg interferon beta‐1b versus 20 mg glatiramer acetate in relapsing‐remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurology 2009;8(10):889‐97. - PubMed
Bornstein 1987 {published data only}
-
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal, Drexler E, et al. A pilot trial of Cop 1 in exacerbating‐remitting multiple sclerosis. New England Journal of Medicine 1987;317(7):408‐14. - PubMed
BRAVO 2014 {published data only}
-
- Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, et al. A randomized placebo‐controlled phase III trial of oral laquinimod for multiple sclerosis. Journal of Neurology 2014;261(4):773‐83. - PubMed
CAMMS223 2008 {published data only}
-
- CAMMS223 Trial Investigators, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. New England Journal of Medicine 2008;359(17):1786‐801. - PubMed
CARE‐MS I 2012 {published data only}
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1819‐28. - PubMed
CARE‐MS II 2012 {published data only}
-
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1829‐39. - PubMed
CombiRx 2013 {published data only}
Comi 2001 {published data only}
-
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double‐blind, randomized, placebo‐controlled study of the effects of glatiramer acetate on magnetic resonance imaging‐‐measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Annals of Neurology 2001;49(3):290‐7. [PUBMED: 11261502] - PubMed
CONFIRM 2012 {published data only}
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo‐controlled phase 3 study of oral BG‐12 or glatiramer in multiple sclerosis. New England Journal of Medicine 2012;367(12):1087‐97. - PubMed
DEFINE 2012 {published data only}
-
- Gold R, Kappos L, Arnold DL, Bar‐Or A, Giovannoni G, Selmaj K, et al. Placebo‐controlled phase 3 study of oral BG‐12 for relapsing multiple sclerosis. New England Journal of Medicine 2012;367(12):1098‐107. - PubMed
Etemadifar 2007 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing‐remitting multiple sclerosis. Journal of Neurology 2007;254(12):1723‐8. - PubMed
EVIDENCE 2007 {published data only}
-
- Schwid S, Panitch H. Full results of the Evidence of Interferon Dose‐Response‐European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor‐blinded comparison of low‐dose weekly versus high‐dose, high‐frequency interferon beta‐1a for relapsing multiple sclerosis. Clinical Therapeutics 2007;29(9):2031‐48. - PubMed
Fazekas 1997 {published data only}
-
- Fazekas F, Deisenhammer F, Strasser‐Fuchs S, Nahler G, Mamoli B. Randomised placebo‐controlled trial of monthly intravenous immunoglobulin therapy in relapsing‐remitting multiple sclerosis. Lancet 1997;349(9052):589‐93. - PubMed
Fazekas 2008 {published data only}
-
- Fazekas F, Lublin F, Li D, Freedman M, Hartung H, Rieckmann P, et al. Intravenous immunoglobulin in relapsing‐remitting multiple sclerosis: a dose‐finding trial. Neurology 2008;71(4):265‐71. - PubMed
FREEDOMS 2010 {published data only}
-
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo‐controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):387‐401. - PubMed
FREEDOMS II 2014 {published data only}
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing‐remitting multiple sclerosis (FREEDOMS II): a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Neurology 2014;13(6):545‐56. - PubMed
GALA 2013 {published data only}
-
- Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, GALA Study Group. Three times weekly glatiramer acetate in relapsing‐remitting multiple sclerosis. Annals of Neurology 2013;73(6):705‐13. - PubMed
Goodkin 1991 {published data only}
-
- Goodkin D, Bailly R, Teetzen M, Hertsgaard D, Beatty W. The efficacy of azathioprine in relapsing‐remitting multiple sclerosis. Neurology 1991;41:20‐5. - PubMed
IFNB MS Group 1993 {published data only}
-
- IFNB MSG. Interferon beta‐1b is effective in relapsing‐remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double‐blind, placebo‐controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993;43(4):655‐61. - PubMed
INCOMIN 2002 {published data only}
-
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, et al. Every‐other‐day interferon beta‐1b versus once‐weekly interferon beta‐1a for multiple sclerosis: results of a 2‐year prospective randomised multicentre study (INCOMIN). Lancet 2002;359:1453‐60. - PubMed
Johnson 1995 {published data only}
-
- Johnson K, Brooks B, Cohen J, Ford C, Goldstein J, Lisak R, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing‐remitting multiple sclerosis: results of a phase III multicenter, double‐blind placebo‐controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45(7):1268‐76. - PubMed
Koch‐Henriksen 2006 {published data only}
-
- Koch‐Henriksen N, Sørensen P, Christensen T, Frederiksen J, Ravnborg M, Jensen K, et al. A randomised study of two interferon‐beta treatments in relapsing‐remitting multiple sclerosis. Neurology 2006;66(7):1056‐60. - PubMed
Lewanska 2002 {published data only}
-
- Lewanska M, Siger‐Zajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. European Journal of Neurology 2002;9(6):565‐72. - PubMed
MAIN TRIAL {published data only}
Millefiorini 1997 {published data only}
-
- Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, et al. Randomized placebo‐controlled trial of mitoxantrone in relapsing‐remitting multiple sclerosis: 24‐month clinical and MRI outcome. Journal of Neurology 1997;244(3):153‐9. - PubMed
MSCRG 1996 {published data only}
-
- Jacobs L, Cookfair D, Rudick R, Herndon R, Richert J, Salazar A, et al. Intramuscular interferon beta‐1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology 1996;39:285‐94. - PubMed
OWIMS 1999 {published data only}
-
- OWIMS. Evidence of interferon beta‐1a dose response in relapsing‐remitting MS: the OWIMS Study. The Once Weekly Interferon for MS Study Group. Neurology 1999;53(4):679‐86. - PubMed
PRISMS 1998 {published data only}
-
- PRISMS. Randomised double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352(9139):1498‐504. - PubMed
REGARD 2008 {published data only}
-
- Mikol D, Barkhof F, Chang P, Coyle P, Jeffery D, Schwid S, et al. Comparison of subcutaneous interferon beta‐1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open‐label trial. Lancet Neurology 2008;7(10):903‐14. - PubMed
SELECT 2013 {published data only}
-
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high‐yield process in relapsing‐remitting multiple sclerosis (SELECT): a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;381(9884):2167‐75. - PubMed
TEMSO 2011 {published data only}
-
- O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. New England Journal of Medicine 2011;365(14):1293‐303. - PubMed
TENERE 2014 {published data only}
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta‐1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Multiple Sclerosis (Houndmills, Basingstoke, England) 2014;20(6):705‐16. - PubMed
TOWER 2014 {published data only}
-
- Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurology 2014;13(3):247‐56. - PubMed
TRASFORMS 2010 {published data only}
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):402‐15. - PubMed
References to studies excluded from this review
ACT 2009 {published data only}
-
- Cohen JA, Imrey PB, Calabresi PA, Edwards KR, Eickenhorst T, Felton WL 3rd, et al. Results of the Avonex Combination Trial (ACT) in relapsing‐remitting MS. Neurology 2009;72(6):535‐41. - PubMed
Ashtari 2011 {published data only}
ATAMS 2014 {published data only}
-
- Kappos L, Hartung HP, Freedman MS, Boyko A, Radü EW, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo‐controlled, double‐blind, phase 2 trial. Lancet Neurology 2014;13(4):353‐63. - PubMed
Calabrese 2012 {published data only}
-
- Calabrese M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Rinaldi F, et al. Effect of disease‐modifying drugs on cortical lesions and atrophy in relapsing‐remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(4):418‐24. - PubMed
CHOICE 2010 {published data only}
-
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double‐blind, placebo‐controlled, add‐on trial with interferon beta. Lancet Neurology 2010;9(4):381‐90. - PubMed
Etemadifar 2006 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing‐remitting multiple sclerosis. Acta Neurologica Scandinavica 2006;113(5):283‐7. - PubMed
FORTE 2011 {published data only}
-
- Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M, FORTE Study Group. Phase III dose‐comparison study of glatiramer acetate for multiple sclerosis. Annals of Neurology 2011;69(1):75‐82. - PubMed
Freedman 2012 {published data only}
-
- Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon‐β in relapsing multiple sclerosis: a randomized phase II trial. Neurology 2012;78(23):1877‐85. - PubMed
Havrdova 2009 {published data only}
-
- Havrdova E, Zivadinov R, Krasensky J, Dwyer MG, Novakova I, Dolezal O, et al. Randomized study of interferon beta‐1a, low‐dose azathioprine, and low‐dose corticosteroids in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2009;15(8):965‐76. - PubMed
Kappos 2006 {published data only}
-
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. New Egyptian Journal of Medicine 2006;355(11):1124‐40. - PubMed
Kappos 2008 {published data only}
-
- Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing‐remitting multiple sclerosis: a multicentre, randomised, double‐blind, placebo‐controlled phase IIb study. Lancet 2008;372(9648):1463‐72. - PubMed
Kappos 2011 {published data only}
-
- Kappos L, Li D, Calabresi PA, O'Connor P, Bar‐Or A, Barkhof F, et al. Ocrelizumab in relapsing‐remitting multiple sclerosis: a phase 2, randomised, placebo‐controlled, multicentre trial. Lancet 2011;378(9805):1779‐87. - PubMed
Khoury 2010 {published data only}
Knobler 1993 {published data only}
-
- Knobler RL, Greenstein JI, Johnson KP, Lublin FD, Panitch HS, Conway K, et al. Systemic recombinant human interferon‐beta treatment of relapsing‐remitting multiple sclerosis: pilot study analysis and six‐year follow‐up. Journal of Interferon Research 1993;13(5):333‐40. - PubMed
Saida 2012 {published data only}
-
- Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(9):1269‐77. - PubMed
SENTINEL 2006 {published data only}
-
- Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta‐1a for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):911‐23. - PubMed
Sorensen 2014 {published data only}
-
- Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing‐remitting multiple sclerosis: a phase 2 study. Neurology 2014;82(7):573‐81. - PubMed
References to ongoing studies
DECIDE {published data only}
-
- Multicenter, double‐blind, randomized, parallel‐group, monotherapy, active‐control study to determine the efficacy and safety of daclizumab high yield process (DAC HYP) versus Avonex® (interferon β 1a) in patients with relapsing‐remitting multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01064401 (accessed 30 September 2014).
NCT01247324 {published data only}
-
- A randomized, double‐blind, double‐dummy, parallel‐group study to evaluate the efficacy and safety of Ocrelizumab in comparison to interferon beta‐1a (Rebif®) in patients with relapsing multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01247324 (accessed 30 September 2014).
NCT01412333 {published data only}
-
- A randomized, double‐blind, double‐dummy, parallel‐group study to evaluate the efficacy and safety of Ocrelizumab in comparison to Interferon Beta‐1a (Rebif®) in patients with relapsing multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01412333 (accessed 30 September 2014).
Additional references
Aharoni 2014
Association of British Neurologists 2005
-
- Association of British Neurologists 2005. Guidelines for the use of intravenous immunoglobulin in neurological diseases. www.theabn.org/documents/IVIg‐Guidelines (accessed October 2013).
Bennett 2009
-
- Bennett J, Stüve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: therapeutic implications. Clinical Neuropharmacology 2009;32(3):121‐32. - PubMed
Birnbaum 2008
-
- Birnbaum G, Cree B, Altafullah I, Zinser M, Reder A. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology 2008;71(18):1390–5. - PubMed
Caldwell 2005
Carroll 2014
-
- Carroll CA, Fairman KA, Lage MJ. Updated cost‐of‐care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Services Research 2014;14:286. [http://www.biomedcentral.com/1472‐6963/14/286] - PMC - PubMed
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. - PubMed
Chaimani 2013
Claussen 2012
-
- Claussen MC, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clinical Immunology 2012;142:49‐56. - PubMed
Compston 2008
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502‐17. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Ebers 2008
-
- Ebers G, Heigenhauser L, Daumer M, Lederer C, Noseworthy J. Disability as an outcome in MS clinical trials. Neurology 2008;71:624–31. - PubMed
Elovaara 2008
-
- Elovaara I, Apostolski S, Doorn P, Gilhus N, Hietaharju A, Honkaniemi J, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. European Journal of Neurology 2008;15(9):893‐908. - PubMed
EMA 2006
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Tysabri. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2011
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Gilenya. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2013a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Aubagio. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2013b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Lemtrada. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info... (accessed June 2014).
EMA 2014a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Tecfidera. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info... (accessed June 2014).
EMA 2014b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Plegridy. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_... (accessed June 2015).
EMA 2014c
-
- European Medicines Agency. Refusal of the marketing authorisation for Nerventra (laquinimod). http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_... (accessed June 2014).
EMEA 1997
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Avonex. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_... (accessed June 2015).
EMEA 1998
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Rebif. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_R... (accessed June 2014).
EMEA 2002
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Betaferon. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
FDA 1993
-
- U.S. Food, Drug Administration. Betaseron interferon beta‐1b subcutaneous. Drug Approval Package ‐ Licensing Action 1993. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103471s5063s506... (accessed June 2014).
FDA 1996
-
- U.S. Food, Drug Administration. Glatiramer acetate (Capoxane) Product Approval Information ‐ Licensing Action 1996. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2000
-
- U.S. Food, Drug Administration. Mitoxantrone (Novantrone) Product Approval Information ‐ Licensing Action 2000. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2002
-
- U.S. Food, Drug Administration. Interferon beta‐1a (Rebif) Product Approval Information ‐ Licensing Action 2002. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDeveloped... (accessed June 2014).
FDA 2003
-
- U.S. Food, Drug Administration. Interferon beta‐1a (Avonex) Product Approval Information ‐ Licensing Action 2003. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/103628s5021TOC.cfm (accessed June 2014).
FDA 2004
-
- U.S. Food, Drug Administration. Tysabri (Natalizumab) Product Approval Information 2004. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/125104s000_Natali... (accessed June 2014).
FDA 2006
-
- U.S. Food, Drug Administration. FDA Approves Resumed Marketing of Tysabri Under a Special Distribution Program. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108662... (accessed June 2014).
FDA 2010
-
- U.S. Food, Drug Administration. Gilenya (Fingolimod) Product Approval Information 2010. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2012
-
- U.S. Food, Drug Administration. Aubagio (Teriflunomide) Product Approval Information 2012. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2013
-
- U.S. Food, Drug Administration. Tecfidera (Dimethyl fumarate) Product Approval Information 2013. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2014a
-
- U.S. Food, Drug Administration. Alemtuzumab (Lemtrada) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/103948Orig1... (accessed Sept 2014).
FDA 2014b
-
- U.S. Food, Drug Administration. Peginterferon beta‐1a (Plegridy) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/biologics/2004/1... (accessed September 2014).
FDA 2014c
-
- U.S. Food, Drug Administration. Imuran Product Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/016324s037,0173... (accessed June 2015).
Filippini 2013
Fox 2004
-
- Fox E. Mechanism of action of mitoxantrone. Neurology 2004;12:15‐8. - PubMed
Glenny 2005
-
- Glenny A, Altman D, Song F, Sakarovitch C, Deeks J, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9:1‐34. - PubMed
Goodin 2002
-
- Goodin DS, Frohman EM, Garmany GP Jr, Halper J, Likosky WH, Lublin FD, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002;58(2):169‐78. - PubMed
Goodman 2009
GRADE Working Group 2004
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
Hadjigeorgiou 2013
-
- Hadjigeorgiou GM, Doxani C, Miligkos M, Ziakas P, Bakalos G, Papadimitriou D, et al. A network meta‐analysis of randomized controlled trials for comparing the effectiveness and safety profile of treatments with marketing authorization for relapsing multiple sclerosis. Journal of Clinical Pharmacy and Therapeutics 2013;38(6):433‐9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
Hill‐Cawthorne 2012
-
- Hill‐Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83:298‐304. - PubMed
Hu 2012
-
- Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, et al. A novel PEGylated interferon beta‐1a for multiple sclerosis: safety, pharmacology, and biology. Journal of Clinical Pharmacology 2012;52:798–808. - PubMed
Inusah 2010
-
- Inusah S, Sormani MP, Cofield SS, Aban IB, Musani SK, Srinivasasainagendra V, et al. Assessing changes in relapse rates in multiple sclerosis. Multiple Sclerosis 2010;16:1414–21. - PubMed
Jackson 2014
Kieseier 2011
-
- Kieseier BC. The mechanism of action of interferon‐β in relapsing multiple sclerosis. CNS Drugs 2011;25:491‐502. - PubMed
Kremenchutzky 2006
-
- Kremenchutzky M, Rice G, Baskerville J, Wingerchuk D, Ebers G. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 2006;129:584‐94. - PubMed
Kurtzke 1983
-
- Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444‐52. - PubMed
Lublin 1996
-
- Lublin F, Reingold S. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907‐11. - PubMed
Mandala 2002
-
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine‐1‐phosphate receptor agonists. Science 2002;296:346‐9. - PubMed
McDonald 2001
-
- McDonald W, Compston A, Edan G, Goodkin D, Hartung H, Lublin F. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50:121‐7. - PubMed
Miladinovic 2014
-
- Miladinovic B, Hozo I, Chaimani A, Djulbegovic B. Indirect treatment comparison. Stata Journal 2014;14(1):76‐86.
Nicholas 2012
-
- Nicholas R, Straube S, Schmidli H, Pfeiffer S, Friede T. Time‐patterns of annualized relapse rates in randomized placebo‐controlled clinical trials in relapsing multiple sclerosis: a systematic review and meta‐analysis. Multiple Sclerosis 2012;18:1290‐6. - PubMed
Oh 2013
-
- Oh J, Calabresi PA. Emerging injectable therapies for multiple sclerosis. Lancet Neurology 2013;12:1115–26. - PubMed
Peters 2008
-
- Peters J, Sutton A, Jones D, Abrams K, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. - PubMed
Polman 2005
-
- Polman C, Reingold S, Edan G, Filippi M, Hartung H, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the 'McDonald Criteria'. Annals of Neurology 2005;58:840‐6. - PubMed
Polman 2011
Poser 1983
-
- Poser C, Paty D, Scheinberg L, McDonald W, Davis F, Ebers G, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227‐31. - PubMed
Ravnborg 2010
-
- Ravnborg M, Sørensen P, Andersson M, Celius E, Jongen P, Elovaara I, et al. Methylprednisolone in combination with interferon beta‐1a for relapsing‐remitting multiple sclerosis (MECOMBIN study): a multicentre, double blind, randomised, placebo‐controlled, parallel‐group trial. Lancet Neurology 2010;9:672‐80. - PubMed
Salanti 2011
-
- Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163‐71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. - PubMed
Salanti 2014
Scalfari 2014
-
- Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long‐term evolution of multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 2014;85:67‐75. - PubMed
Schmied 2003
-
- Schmied M, Duda PW, Krieger JI, Trollmo C, Hafler DA. In vitro evidence that subcutaneous administration of glatiramer acetate induces hyporesponsive T cells in patients with multiple sclerosis. Clinical Immunology 2003;106:163‐74. - PubMed
Schünemann 2011
-
- Schünemann H, Oxman A, Higgins J, Vist G, Glasziou P, Guyatt G. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stangel 1999
-
- Stangel M, Toyka K, Gold R. Mechanisms of high‐dose intravenous immunoglobulins in demyelinating diseases. Archives of Neurology 1999;56(6):661‐3. - PubMed
Steinvorth 2013
-
- Steinvorth SM, Röver C, Schneider S, Nicholas R, Straube S, Friede T. Explaining temporal trends in annualised relapse rates in placebo groups of randomised controlled trials in relapsing multiple sclerosis: systematic review and meta‐regression. Multiple Sclerosis 2013;19:1580‐6. - PubMed
Stellmann 2012
-
- Stellmann JP, Neuhaus A, Herich L, Schippling S, Roeckel M, Daumer M, et al. Placebo cohorts in phase‐3 MS treatment trials, predictors for on‐trial disease activity 1990‐2010 based on a meta‐analysis and individual case data. PLoS One 2012;7(11):e50347. [DOI: 10.1371/journal.pone.0050347] - DOI - PMC - PubMed
Tiede 2003
Varrin‐Doyer 2014
Veroniki 2013
Weiner 1993
-
- Weiner H, Mackin G, Matsui M, Orav E, Khoury S, Dawson D, et al. Double‐blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 1993;259(5099):1321–4. - PubMed
White 2011
-
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11:255‐70.
White 2012
Wilms 2010
-
- Wilms H, Sievers J, Rickert U, Rostami‐Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL‐1beta, TNF‐alpha and IL‐6 in an in‐vitro model of brain inflammation. Journal of Neuroinflammation 2010;7:30. - PMC - PubMed
Wuest 2011
Yednock 1992
-
- Yednock TA, Cannon C, Fritz LC, Sanchez‐Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992;356:63‐6. - PubMed
Yousry 2006
Zintzaras 2012
-
- Zintzaras E, Doxani C, Mprotsis T, Schmid CH, Hadjigeorgiou G. Network analysis of randomized controlled trials in multiple sclerosis. Clinical Therapeutics 2012;34(4):857–69. - PubMed
References to other published versions of this review
Tramacere 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

