Patient-Derived Xenografts as a Model System for Radiation Research
- PMID: 26384275
- PMCID: PMC4758448
- DOI: 10.1016/j.semradonc.2015.05.008
Patient-Derived Xenografts as a Model System for Radiation Research
Abstract
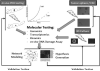
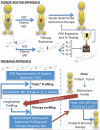
The cancer literature is filled with promising preclinical studies demonstrating impressive efficacy for new therapeutics, yet translation of these approaches into clinical successes has been rare, indicating that current methods used to predict efficacy are suboptimal. The most likely reason for the limitation of these studies is the disconnect between preclinical models and cancers treated in the clinic. Specifically, most preclinical models are poor representations of human disease. Immortalized cancer cell lines that dominate the cancer literature may be, in a sense, "paper tigers" that have been selected by decades of culture to be artificially driven by highly targetable proteins. Thus, although effective in treating these cell lines either in vitro or as artificial tumors transplanted from culture into experimental animals as xenografts, the identified therapies would likely underperform in a clinical setting. This inherent limitation applies not only to drug testing but also to experiments with radiation therapy. Indeed, traditional radiobiology methods rely on monolayer culture systems, with emphasis on colony formation and DNA damage assessment that may have limited clinical translation. As such, there has been keen interest in developing tumor explant systems in which patient tumors are directly transplanted into and solely maintained in vivo, using immunocompromised mice. These so-called patient-derived xenografts (PDXs) represent a robust model system that has been garnering support in academia and industry as a superior preclinical approach to drug testing. Likewise, PDX models have the potential to improve radiation research. In this review, we describe how PDX models are currently being used for both drug and radiation testing and how they can be incorporated into a translational research program.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. - PMC - PubMed
-
- Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Invest. 2013;93:970–982. - PubMed
-
- Choi SY, Lin D, Gout PW, Collins CC, Xu Y, Wang Y. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev. 2014;79-80C:222–237. - PubMed
-
- Bredel M, Jacoby E. Chemogenomics: an emerging strategy for rapid target and drug discovery. Nat Rev Genet. 2004;5:262–275. - PubMed
-
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

