A novel human truncated IL12rβ1-Fc fusion protein ameliorates experimental autoimmune encephalomyelitis via specific binding of p40 to inhibit Th1 and Th17 cell differentiation
- PMID: 26384304
- PMCID: PMC4745676
- DOI: 10.18632/oncotarget.5164
A novel human truncated IL12rβ1-Fc fusion protein ameliorates experimental autoimmune encephalomyelitis via specific binding of p40 to inhibit Th1 and Th17 cell differentiation
Abstract
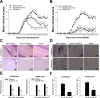
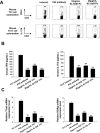
Interleukin (IL)-12 and IL-23 respectively driving polarization of T helper (Th) 1 and Th17 cells has been strongly implicated in the pathogenesis of both multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). In this study, we first constructed, expressed and purified a novel human truncated IL12rβ1-Fc fusion protein (tIL12rβ1/Fc) binding multiple forms of the p40 subunit of human IL-12 and IL-23. tIL12rβ1/Fc was found to effectively ameliorate MOG35-55-induced EAE through reducing the production of Th1- and Th17-polarized pro-inflammatory cytokines and suppressing inflammation and demyelination in the focused parts. Moreover, tIL12rβ1/Fc suppressed Th1 (IFN-γ(+) alone) and IFN-γ(+) IL-17(+) as well as the population of classic Th17 (IL-17(+) alone) cells in vivo. Furthermore, tIL12rβ1/Fc ameliorated EAE at the peak of disease via the inhibition of STAT pathway, thereby causing a prominent reduction of RORγt (Th17) and T-bet (Th1) expression. Notably, tIL12rβ1/Fc could increase the relative number of CD4(+) Foxp3(+) regulatory T cells. These findings indicates that tIL12rβ1/Fc is a novel fusion protein for specific binding multiple forms of p40 subunit to exert potent anti-inflammatory effects and provides a valuable approach for the treatment of MS and other autoimmune diseases.
Keywords: Immune response; Immunity; Immunology and Microbiology Section; autoimmune disease; binding specificity; central nervous system; cytokine-blocking; molecular mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Dehydrodiconiferyl alcohol (DHCA) modulates the differentiation of Th17 and Th1 cells and suppresses experimental autoimmune encephalomyelitis.Mol Immunol. 2015 Dec;68(2 Pt B):434-44. doi: 10.1016/j.molimm.2015.09.028. Epub 2015 Oct 23. Mol Immunol. 2015. PMID: 26477735
-
Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway.Sci Rep. 2015 Nov 30;5:17407. doi: 10.1038/srep17407. Sci Rep. 2015. PMID: 26616302 Free PMC article.
-
Arctigenin Suppress Th17 Cells and Ameliorates Experimental Autoimmune Encephalomyelitis Through AMPK and PPAR-γ/ROR-γt Signaling.Mol Neurobiol. 2016 Oct;53(8):5356-66. doi: 10.1007/s12035-015-9462-1. Epub 2015 Oct 6. Mol Neurobiol. 2016. PMID: 26440666
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Th17 Cells and autoimmune encephalomyelitis (EAE/MS).Allergol Int. 2008 Jun;57(2):115-20. doi: 10.2332/allergolint.R-07-159. Allergol Int. 2008. PMID: 18427164 Review.
Cited by
-
Anti-inflammatory effects of interleukin-23 receptor cytokine-binding homology region rebalance T cell distribution in rodent collagen-induced arthritis.Oncotarget. 2016 May 31;7(22):31800-13. doi: 10.18632/oncotarget.9309. Oncotarget. 2016. PMID: 27177334 Free PMC article.
-
Human IL-23R Cytokine-Binding Homology Region-Fc Fusion Protein Ameliorates Psoriasis via the Decrease of Systemic Th17 and ILC3 Cell Responses.Int J Mol Sci. 2019 Aug 26;20(17):4170. doi: 10.3390/ijms20174170. Int J Mol Sci. 2019. PMID: 31454926 Free PMC article.
-
CD4+T cell specific B7-H1 selectively inhibits proliferation of naïve T cells and Th17 differentiation in experimental autoimmune encephalomyelitis.Oncotarget. 2017 Sep 28;8(52):90028-90036. doi: 10.18632/oncotarget.21357. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163808 Free PMC article.
-
Systemic exposure to a single dose of ferucarbotran aggravates neuroinflammation in a murine model of experimental autoimmune encephalomyelitis.Int J Nanomedicine. 2019 Feb 15;14:1229-1240. doi: 10.2147/IJN.S189327. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30863056 Free PMC article.
References
-
- Costa GL, Sandora MR, Nakajima A, Nguyen EV, Taylor-Edwards C, Slavin AJ, Contag CH, Fathman CG, Benson JM. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. The Journal of Immunology. 2001;167:2379–2387. - PubMed
-
- Moldovan IR, Rudick RA, Cotleur AC, Born SE, Lee J-C, Karafa MT, Pelfrey CM. Interferon gamma responses to myelin peptides in multiple sclerosis correlate with a new clinical measure of disease progression. Journal of neuroimmunology. 2003;141:132–140. - PubMed
-
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature medicine. 2007;13:139–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

