Metabolic engineering of the mixed-acid fermentation pathway of Escherichia coli for anaerobic production of glutamate and itaconate
- PMID: 26384341
- PMCID: PMC4573741
- DOI: 10.1186/s13568-015-0147-y
Metabolic engineering of the mixed-acid fermentation pathway of Escherichia coli for anaerobic production of glutamate and itaconate
Abstract
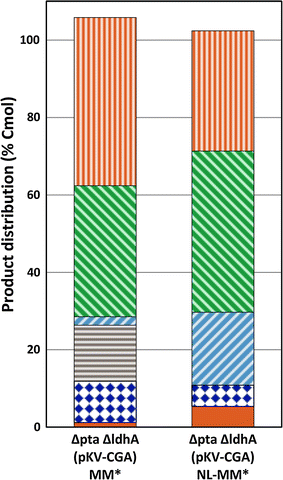
Itaconic acid, an unsaturated C5-dicarboxylic acid, is a biobased building block for the polymer industry. The purpose of this study was to establish proof of principle for an anaerobic fermentation process for the production of itaconic acid by modification of the mixed acid fermentation pathway of E. coli. E. coli BW25113 (DE3) and the phosphate acetyltransferase (pta) and lactate dehydrogenase (ldhA) deficient strain E. coli BW25113 (DE3) Δpta-ΔldhA were used to study anaerobic itaconate production in E. coli. Heterologous expression of the gene encoding cis-aconitate decarboxylase (cadA) from A. terreus in E. coli BW25113 (DE3) did not result in itaconate production under anaerobic conditions, but 0.08 mM of itaconate was formed when the genes encoding citrate synthase (gltA) and aconitase (acnA) from Corynebacterium glutamicum were also expressed. The same amount was produced when cadA was expressed in E. coli BW25113 (DE3) Δpta-ΔldhA. The titre increased 8 times to 0.66 mM (1.2 % Cmol) when E. coli BW25113 (DE3) Δpta-ΔldhA also expressed gltA and acnA. In addition, this strain produced 8.5 mM (13 % Cmol) of glutamate. The use of a nitrogen-limited growth medium reduced the accumulation of glutamate by nearly 50 % compared to the normal medium, and also resulted in a more than 3-fold increase of the itaconate titre to 2.9 mM. These results demonstrated that E. coli has potential to produce itaconate and glutamate under anaerobic conditions, closing the redox balance by co-production of succinate or ethanol with H2 and CO2.
Keywords: Anaerobic fermentation; Escherichia coli; Ethanol; Glutamic acid; Itaconic acid; Metabolic engineering; Redox balance.
Figures



References
-
- Clark DP. The fermentation pathways of Escherichia coli fems. Microbiol Rev. 1989;63(3):223–234. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

