Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK
- PMID: 26384382
- PMCID: PMC4686944
- DOI: 10.2337/db15-0647
Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK
Abstract
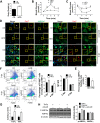
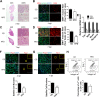
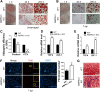
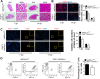
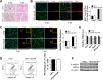
Obesity is increasing rapidly worldwide and is accompanied by many complications, including impaired muscle regeneration. The obese condition is known to inhibit AMPK activity in multiple tissues. We hypothesized that the loss of AMPK activity is a major reason for hampered muscle regeneration in obese subjects. We found that obesity inhibits AMPK activity in regenerating muscle, which was associated with impeded satellite cell activation and impaired muscle regeneration. To test the mediatory role of AMPKα1, we knocked out AMPKα1 and found that both proliferation and differentiation of satellite cells are reduced after injury and that muscle regeneration is severely impeded, reminiscent of hampered muscle regeneration seen in obese subjects. Transplanted satellite cells with AMPKα1 deficiency had severely impaired myogenic capacity in regenerating muscle fibers. We also found that attenuated muscle regeneration in obese mice is rescued by AICAR, a drug that specifically activates AMPK, but AICAR treatment failed to improve muscle regeneration in obese mice with satellite cell-specific AMPKα1 knockout, demonstrating the importance of AMPKα1 in satellite cell activation and muscle regeneration. In summary, AMPKα1 is a key mediator linking obesity and impaired muscle regeneration, providing a convenient drug target to facilitate muscle regeneration in obese populations.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures



References
-
- Kennedy JW, Hirshman MF, Gervino EV, et al. . Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999;48:1192–1197 - PubMed
-
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med 1991;12:184–207 - PubMed
-
- Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol (1985) 1987;63:1816–1821 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

