Guanine quadruplex structures localize to heterochromatin
- PMID: 26384414
- PMCID: PMC4705689
- DOI: 10.1093/nar/gkv900
Guanine quadruplex structures localize to heterochromatin
Erratum in
-
Guanine quadruplex structures localize to heterochromatin.Nucleic Acids Res. 2017 Jun 2;45(10):6253. doi: 10.1093/nar/gkx301. Nucleic Acids Res. 2017. PMID: 28449026 Free PMC article. No abstract available.
Abstract
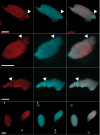
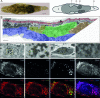
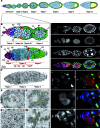
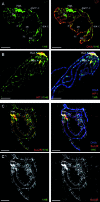
Increasing amounts of data support a role for guanine quadruplex (G4) DNA and RNA structures in various cellular processes. We stained different organisms with monoclonal antibody 1H6 specific for G4 DNA. Strikingly, immuno-electron microscopy showed exquisite specificity for heterochromatin. Polytene chromosomes from Drosophila salivary glands showed bands that co-localized with heterochromatin proteins HP1 and the SNF2 domain-containing protein SUUR. Staining was retained in SUUR knock-out mutants but lost upon overexpression of SUUR. Somatic cells in Macrostomum lignano were strongly labeled, but pluripotent stem cells labeled weakly. Similarly, germline stem cells in Drosophila ovaries were weakly labeled compared to most other cells. The unexpected presence of G4 structures in heterochromatin and the difference in G4 staining between somatic cells and stem cells with germline DNA in ciliates, flatworms, flies and mammals point to a conserved role for G4 structures in nuclear organization and cellular differentiation.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Guanine quadruplex monoclonal antibody 1H6 cross-reacts with restrained thymidine-rich single stranded DNA.Nucleic Acids Res. 2017 Jun 2;45(10):5913-5919. doi: 10.1093/nar/gkx245. Nucleic Acids Res. 2017. PMID: 28449085 Free PMC article.
-
Detection of G-quadruplex DNA in mammalian cells.Nucleic Acids Res. 2014 Jan;42(2):860-9. doi: 10.1093/nar/gkt957. Epub 2013 Oct 24. Nucleic Acids Res. 2014. PMID: 24163102 Free PMC article.
-
[Species-specific localization of DNA from pericentromeric heterochromatin on polytene chromosomes in the salivary gland cells and 3D-nuclear organization nurse cells in Drosophila virilis, and Drosophila kanekoi (Diptera: Drosophilidae)].Genetika. 2014 Nov;50(11):1299-304. Genetika. 2014. PMID: 25739282 Russian.
-
Intercalary heterochromatin and genetic silencing.Bioessays. 2003 Nov;25(11):1040-51. doi: 10.1002/bies.10343. Bioessays. 2003. PMID: 14579245 Review.
-
Histone modification and the control of heterochromatic gene silencing in Drosophila.Chromosome Res. 2006;14(4):377-92. doi: 10.1007/s10577-006-1066-1. Chromosome Res. 2006. PMID: 16821134 Review.
Cited by
-
Spontaneous DNA Synapsis by Forming Noncanonical Intermolecular Structures.Polymers (Basel). 2022 May 23;14(10):2118. doi: 10.3390/polym14102118. Polymers (Basel). 2022. PMID: 35632001 Free PMC article.
-
G-quadruplex structures mark human regulatory chromatin.Nat Genet. 2016 Oct;48(10):1267-72. doi: 10.1038/ng.3662. Epub 2016 Sep 12. Nat Genet. 2016. PMID: 27618450
-
Fundamentals of G-quadruplex biology.Annu Rep Med Chem. 2020;54:3-44. doi: 10.1016/bs.armc.2020.06.004. Epub 2020 Jul 30. Annu Rep Med Chem. 2020. PMID: 32836507 Free PMC article.
-
Integrative characterization of G-Quadruplexes in the three-dimensional chromatin structure.Epigenetics. 2019 Sep;14(9):894-911. doi: 10.1080/15592294.2019.1621140. Epub 2019 Jun 10. Epigenetics. 2019. PMID: 31177910 Free PMC article.
-
Telomeric retrotransposons show propensity to form G-quadruplexes in various eukaryotic species.Mob DNA. 2023 Apr 10;14(1):3. doi: 10.1186/s13100-023-00291-9. Mob DNA. 2023. PMID: 37038191 Free PMC article.
References
-
- Bickmore W.A., van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell. 2013;152:1270–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

