No peroxisome is an island - Peroxisome contact sites
- PMID: 26384874
- PMCID: PMC4869879
- DOI: 10.1016/j.bbamcr.2015.09.016
No peroxisome is an island - Peroxisome contact sites
Abstract
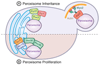
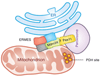
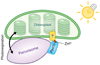
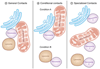
In order to optimize their multiple cellular functions, peroxisomes must collaborate and communicate with the surrounding organelles. A common way of communication between organelles is through physical membrane contact sites where membranes of two organelles are tethered, facilitating exchange of small molecules and intracellular signaling. In addition contact sites are important for controlling processes such as metabolism, organelle trafficking, inheritance and division. How peroxisomes rely on contact sites for their various cellular activities is only recently starting to be appreciated and explored and the extent of peroxisomal communication, their contact sites and their functions are less characterized. In this review we summarize the identified peroxisomal contact sites, their tethering complexes and their potential physiological roles. Additionally, we highlight some of the preliminary evidence that exists in the field for unexplored peroxisomal contact sites.
Keywords: Chloroplast; Endoplasmic reticulum; Lipid droplet; Membrane contact site; Mitochondria; Peroxisome.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

