Targeting IL4/IL4R for the treatment of epithelial cancer metastasis
- PMID: 26385103
- PMCID: PMC4651701
- DOI: 10.1007/s10585-015-9747-9
Targeting IL4/IL4R for the treatment of epithelial cancer metastasis
Abstract
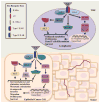
While progress has been made in treating primary epithelial tumors, metastatic tumors remain largely incurable and still account for 85-90 % of all cancer-related deaths. Interleukin-4 (IL4), a Th2 cytokine, and the IL4/IL4 receptor (IL4R) interaction have well defined roles in the immune system. Yet, IL4 receptors are over-expressed by many epithelial cancers and could be a promising target for metastatic tumor therapy. The IL4/IL4R signaling axis is a strong promoter of pro-metastatic phenotypes in epithelial cancer cells including enhanced migration, invasion, survival, and proliferation. The promotion of breast cancer growth specifically is also supported in part by IL4-induced glutamine metabolism, and we have shown that IL4 is also capable of inducing glucose metabolism in breast cancer cells. Importantly, there are several types of FDA approved medications for use in asthma patients that inhibit the IL4/IL4R signaling axis. However, these approved medications inhibit both the type I IL4 receptor found on immune cells, and the type II IL4 receptor that is predominantly expressed by some non-hematopoietic cells including epithelial cancer cells. This article reviews existing therapies targeting IL4, IL4R, or IL4/IL4R signaling, and recent findings guiding the creation of novel therapies that specifically inhibit the type II IL4R, while taking into consideration effects on immune cells within the tumor microenvironment. Some of these therapies are currently in clinical trials for cancer patients, and may be exploitable for the treatment of metastatic disease.
Keywords: Cytokine; IL4; IL4 receptor; Metastasis; Proliferation; Survival.
Conflict of interest statement
No conflicts of interest to declare.
Figures
Similar articles
-
IL4 receptor α mediates enhanced glucose and glutamine metabolism to support breast cancer growth.Biochim Biophys Acta. 2015 May;1853(5):1219-28. doi: 10.1016/j.bbamcr.2015.02.020. Epub 2015 Mar 4. Biochim Biophys Acta. 2015. PMID: 25746764 Free PMC article.
-
Interleukin-13 is a potent activator of JAK3 and STAT6 in cells expressing interleukin-2 receptor-gamma and interleukin-4 receptor-alpha.Biochem J. 1996 Nov 1;319 ( Pt 3)(Pt 3):865-72. doi: 10.1042/bj3190865. Biochem J. 1996. PMID: 8920992 Free PMC article.
-
IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis.Theranostics. 2024 Apr 8;14(6):2605-2621. doi: 10.7150/thno.92672. eCollection 2024. Theranostics. 2024. PMID: 38646639 Free PMC article.
-
[Inflammatory cytokines (IL-4, IL-5 and IL-13)].Nihon Rinsho. 2001 Oct;59(10):1894-9. Nihon Rinsho. 2001. PMID: 11676128 Review. Japanese.
-
Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13.Cancer Res. 2012 Dec 15;72(24):6338-43. doi: 10.1158/0008-5472.CAN-12-3544. Epub 2012 Dec 7. Cancer Res. 2012. PMID: 23222300 Free PMC article. Review.
Cited by
-
Association Between Promoter Polymorphisms of IL-1B, IL-4 and IL-6 Genes and a Viral Load Infected Women with Human Papillomavirus.J Reprod Infertil. 2021 Apr-Jun;22(2):92-102. doi: 10.18502/jri.v22i2.5794. J Reprod Infertil. 2021. PMID: 34041005 Free PMC article.
-
Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy.Front Immunol. 2021 May 3;12:669474. doi: 10.3389/fimmu.2021.669474. eCollection 2021. Front Immunol. 2021. PMID: 34012451 Free PMC article. Review.
-
Scientific reports concerning the impact of interleukin 4, interleukin 10 and transforming growth factor β on cancer cells.Cent Eur J Immunol. 2019;44(2):190-200. doi: 10.5114/ceji.2018.76273. Epub 2019 Jul 30. Cent Eur J Immunol. 2019. PMID: 31530989 Free PMC article. Review.
-
Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy.Cell Oncol (Dordr). 2022 Jun;45(3):333-353. doi: 10.1007/s13402-022-00667-8. Epub 2022 May 19. Cell Oncol (Dordr). 2022. PMID: 35587857 Review.
-
Oncogenic KRAS-Driven Metabolic Reprogramming in Pancreatic Cancer Cells Utilizes Cytokines from the Tumor Microenvironment.Cancer Discov. 2020 Apr;10(4):608-625. doi: 10.1158/2159-8290.CD-19-0297. Epub 2020 Feb 11. Cancer Discov. 2020. PMID: 32046984 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

