Synthesis of ent-ketorfanol via a C-H alkenylation/torquoselective 6π electrocyclization cascade
- PMID: 26385263
- PMCID: PMC4676713
- DOI: 10.1002/anie.201505604
Synthesis of ent-ketorfanol via a C-H alkenylation/torquoselective 6π electrocyclization cascade
Abstract
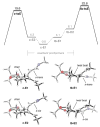
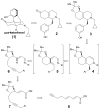
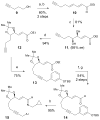
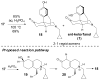
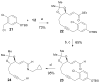
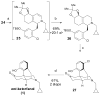
The asymmetric synthesis of ent-ketorfanol from simple and commercially available precursors is reported. A Rh(I) -catalyzed intramolecular CH alkenylation/torquoselective 6π electrocyclization cascade provides a fused bicyclic 1,2-dihydropyridine as a key intermediate. Computational studies were performed to understand the high torquoselectivity of the key 6π electrocyclization. The computational results demonstrate that a conformational effect is responsible for the observed selectivity. The ketone functionality and final ring are introduced in a single step by a redox-neutral acid-catalyzed rearrangement of a vicinal diol to give the requisite carbonyl, followed by intramolecular Friedel-Crafts alkylation.
Keywords: CH activation; alkaloids; asymmetric synthesis; heterocycles; torquoselectivity.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
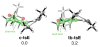
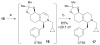
Similar articles
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
-
Expedient Access to 2,3-Dihydropyridines from Unsaturated Oximes by Rh(III)-Catalyzed C-H Activation.J Am Chem Soc. 2015 Jul 22;137(28):8892-5. doi: 10.1021/jacs.5b04946. Epub 2015 Jul 8. J Am Chem Soc. 2015. PMID: 26154248 Free PMC article.
-
Rh(III)-catalyzed intramolecular redox-neutral cyclization of alkenes via C-H activation.Chem Commun (Camb). 2014 Mar 11;50(20):2650-2. doi: 10.1039/c4cc00029c. Chem Commun (Camb). 2014. PMID: 24468699
-
Rhodium(II)-catalyzed nondirected oxidative alkenylation of arenes: arene loading at one equivalent.Angew Chem Int Ed Engl. 2014 Mar 3;53(10):2683-6. doi: 10.1002/anie.201310539. Epub 2014 Jan 30. Angew Chem Int Ed Engl. 2014. PMID: 24481783
-
Convergent Synthesis of Diverse Tetrahydropyridines via Rh(I)-Catalyzed C-H Functionalization Sequences.Org Process Res Dev. 2014 Sep 19;18(9):1097-1104. doi: 10.1021/op500224x. Epub 2014 Aug 29. Org Process Res Dev. 2014. PMID: 25288871 Free PMC article. Review.
Cited by
-
Gadolinium-based MRI contrast agent for the detection of tyrosinase.Analyst. 2020 Feb 17;145(4):1169-1173. doi: 10.1039/c9an02213a. Analyst. 2020. PMID: 31872821 Free PMC article.
-
Asymmetric synthesis of (-)-naltrexone.Chem Sci. 2018 Oct 23;10(2):535-541. doi: 10.1039/c8sc03748e. eCollection 2019 Jan 14. Chem Sci. 2018. PMID: 30713650 Free PMC article.
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
-
Total Synthesis of (+)-Granatumine A and Related Bislactone Limonoid Alkaloids via a Pyran to Pyridine Interconversion.J Am Chem Soc. 2019 Jun 12;141(23):9191-9196. doi: 10.1021/jacs.9b04508. Epub 2019 Jun 3. J Am Chem Soc. 2019. PMID: 31117671 Free PMC article.
-
Unified Total Synthesis of the Limonoid Alkaloids: Strategies for the De Novo Synthesis of Highly Substituted Pyridine Scaffolds.Chem. 2022 Oct 13;8(10):2856-2887. doi: 10.1016/j.chempr.2022.09.012. Epub 2022 Oct 4. Chem. 2022. PMID: 37396824 Free PMC article.
References
-
-
Typing the name of these drug and drug candidates into the NCGC Pharmaceutical Collection database provides compound structure, bioactivity, full list of literature, and access to ongoing clinical trials, applications, and usage.
-
-
- Filizola M, Devi LA. Nature. 2012;485:314–317. - PMC - PubMed
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Nature. 2012;485:321–326. - PMC - PubMed
- Wu HX, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Nature. 2012;485:327–332. - PMC - PubMed
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Nature. 2012;485:400–404. - PMC - PubMed
-
-
For the synthesis of ketorfanol from the degradation of heavily functionalized natural product starting materials, see: Manmade A, Dalzell HC, Howes JF, Razdan RK. J Med Chem. 1981;24:1437–1440.Ida Y, Nemoto T, Hirayama S, Fujii H, Osa Y, Imai M, Nakamura T, Kanemasa T, Kato A, Nagase H. Bioorg Med Chem. 2012;20:949–961.
-
-
-
For reviews and leading references on syntheses of morphine and related natural products, see: Reed JW, Hudlicky T. Acc Chem Res. 2015;48:674–687.Rinner U, Hudlicky T. Top Curr Chem. 2012;300:33–66.Chida N. Top Curr Chem. 2011;299:1–28.Tissot M, Phipps RJ, Lucas C, Leon RM, Pace RDM, Ngouansavanh T, Gaunt MJ. Angew Chem Int Ed. 2014;53:13498–13501.Angew Chem. 2014;126:13716–13719.Ichiki M, Tanimoto H, Miwa S, Saito R, Sato T, Chida N. Chem Eur J. 2013;19:264–269.Li J, Liu GL, Zhao XH, Du JY, Qu H, Chu WD, Ding M, Jin CY, Wei MX, Fan CA. Chem Asian J. 2013;8:1105–1109.Varghese V, Hudlicky T. Angew Chem Int Ed. 2014;53:4355–4358.Angew Chem. 2014;126:4444–4447.Kimishima A, Umihara H, Mizoguchi A, Yokoshima S, Fukuyama T. Org Lett. 2014;16:6244–6247.
-
-
- Colby DA, Bergman RG, Ellman JA. J Am Chem Soc. 2008;130:3645–3651. - PMC - PubMed
- Duttwyler S, Lu C, Rheingold AL, Bergman RG, Ellman JA. J Am Chem Soc. 2012;134:4064–4067. - PMC - PubMed
- Duttwyler S, Chen S, Takase MK, Wiberg KB, Bergman RG, Ellman JA. Science. 2013;339:678–682. - PMC - PubMed
- Ischay MA, Takase MK, Bergman RG, Ellman JA. J Am Chem Soc. 2013;135:2478–2481. - PMC - PubMed
- Duttwyler S, Chen S, Lu C, Mercado BQ, Bergman RG, Ellman JA. Angew Chem Int Ed. 2014;53:3877–3880. - PMC - PubMed
- Angew Chem. 2014;126:3958–3961.
- Mesganaw T, Ellman JA. Org Process Res Dev. 2014;18:1097–1104. - PMC - PubMed
- Mesganaw T, Ellman JA. Org Process Res Dev. 2014;18:1105–1109. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

