Exploratory phase II trial in a multicenter setting to evaluate the clinical value of a chemosensitivity test in patients with gastric cancer (JACCRO-GC 04, Kubota memorial trial)
- PMID: 26385385
- PMCID: PMC4824809
- DOI: 10.1007/s10120-015-0506-z
Exploratory phase II trial in a multicenter setting to evaluate the clinical value of a chemosensitivity test in patients with gastric cancer (JACCRO-GC 04, Kubota memorial trial)
Abstract
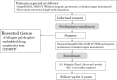
Background: Although postoperative adjuvant chemotherapy with S-1, an oral fluoropyrimidine, has become a standard of care for gastric cancer in Japan, nonresponders may suffer from the cost and adverse reactions without clinical benefit. This multicenter exploratory phase II trial was conducted to see whether a chemosensitivity test, the collagen gel droplet embedded culture drug sensitivity test (CD-DST), can adequately select patients for chemotherapy.
Methods: The CD-DST using four different concentrations of 5-fluorouracil was conducted with resected specimens from preregistered patients who underwent gastrectomy with D2 or more extensive lymphadenectomy. Patients who were histopathologically confirmed to have stage II or greater disease without distant metastasis were eligible for final enrollment. All patients underwent protocol-specified adjuvant chemotherapy with S-1. Three-year relapse-free survival was compared between patients determined as sensitive by the CD-DST (responders) and those deemed insensitive (nonresponders). Appropriate cutoff values for in vitro growth inhibition were defined when the hazard ratio for relapse in responders and the log-rank P values were at their minimum.
Results: Of the 311 patients enrolled, 14 were ineligible and 27 failed to start the protocol treatment. The CD-DST failed in 64 other patients, and survival analyses were conducted with the remaining 206 patients (39 stage II disease, 155 stage III disease, and 12 stage IV disease). The outcome of patients who were determined to be responders was significantly superior to that of nonresponders regardless of the 5-fluorouracil concentrations, although no differences in clinicopathologic characteristics were observed between the two groups, except for age.
Conclusions: The CD-DST identified those who benefit from adjuvant chemotherapy. It deserves further evaluation in the setting of a prospective randomized trial. ClinicalTrials.gov identifier: NCT00287755.
Keywords: Appropriate cutoff values; Chemosensitivity test; Nonresponder; Relapse-free survival; Responder.
Figures



Similar articles
-
Collagen gel droplet-embedded culture drug sensitivity test (CD-DST) predicts the effect of adjuvant chemotherapy on pancreatic cancer.Surg Today. 2019 Dec;49(12):1035-1043. doi: 10.1007/s00595-019-01842-5. Epub 2019 Jul 2. Surg Today. 2019. PMID: 31267224
-
Chemosensitivity test for 5-fluorouracil and 5-chloro-2, 4-dihydroxypyridine predicts outcome of gastric cancer patients receiving S-1 postoperatively.Gastric Cancer. 2010 Nov;13(4):231-7. doi: 10.1007/s10120-010-0564-1. Epub 2010 Dec 3. Gastric Cancer. 2010. PMID: 21128058
-
Stratified phase II trial to establish the usefulness of the collagen gel droplet embedded culture-drug sensitivity test (CD-DST) for advanced gastric cancer.Gastric Cancer. 2014 Oct;17(4):630-7. doi: 10.1007/s10120-013-0320-4. Epub 2013 Dec 8. Gastric Cancer. 2014. PMID: 24318670 Free PMC article. Clinical Trial.
-
Advances in the treatment of gastric cancer.Curr Opin Gastroenterol. 2018 Nov;34(6):465-468. doi: 10.1097/MOG.0000000000000475. Curr Opin Gastroenterol. 2018. PMID: 30303856 Review.
-
Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness.Recent Results Cancer Res. 2003;161:48-61. doi: 10.1007/978-3-642-19022-3_5. Recent Results Cancer Res. 2003. PMID: 12528798 Review.
Cited by
-
Benefits of functional assays in personalized cancer medicine: more than just a proof-of-concept.Theranostics. 2021 Sep 21;11(19):9538-9556. doi: 10.7150/thno.55954. eCollection 2021. Theranostics. 2021. PMID: 34646385 Free PMC article. Review.
-
A PDX model combined with CD-DST assay to evaluate the antitumor properties of KRpep-2d and oxaliplatin in KRAS (G12D) mutant colorectal cancer.Heliyon. 2022 Dec 21;8(12):e12518. doi: 10.1016/j.heliyon.2022.e12518. eCollection 2022 Dec. Heliyon. 2022. PMID: 36590511 Free PMC article.
-
Patient-derived tumor spheroid cultures as a promising tool to assist personalized therapeutic decisions in breast cancer.Transl Cancer Res. 2022 Jan;11(1):134-147. doi: 10.21037/tcr-21-1577. Transl Cancer Res. 2022. PMID: 35261891 Free PMC article.
-
Novel candidate factors predicting the effect of S-1 adjuvant chemotherapy of pancreatic cancer.Sci Rep. 2021 Mar 22;11(1):6541. doi: 10.1038/s41598-021-86099-0. Sci Rep. 2021. PMID: 33753854 Free PMC article.
-
Impact of in vitro chemosensitivity test-guided platinum-based adjuvant chemotherapy on the surgical outcomes of patients with p-stage IIIA non-small cell lung cancer that underwent complete resection.Mol Clin Oncol. 2017 Sep;7(3):327-335. doi: 10.3892/mco.2017.1340. Epub 2017 Jul 24. Mol Clin Oncol. 2017. PMID: 28811897 Free PMC article.
References
-
- Sasako M, Sakuramoto S, Takai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. - DOI - PubMed
-
- Tanigawa N, Mizuno Y, Hashimura T, Honda K, Satomura K, Hikasa Y, et al. Comparison of drug sensitivity among tumor cells within a tumor, between primary and metastases, and between different metastases in the human tumor-colony forming assay. Cancer Res. 1984;44:2309–2312. - PubMed
-
- Trope C, Hakansson L, Dencker H. Heterogeneity of human adenocarcinomas of the colon and the stomach as regards sensitivity to cytostatic drugs. Neoplasma. 1975;22:423–430. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

