Lung-derived exosome uptake into and epigenetic modulation of marrow progenitor/stem and differentiated cells
- PMID: 26385657
- PMCID: PMC4575417
- DOI: 10.3402/jev.v4.26166
Lung-derived exosome uptake into and epigenetic modulation of marrow progenitor/stem and differentiated cells
Abstract
Background: Our group has previously demonstrated that murine whole bone marrow cells (WBM) that internalize lung-derived extracellular vesicles (LDEVs) in culture express pulmonary epithelial cell-specific genes for up to 12 weeks. In addition, the lungs of lethally irradiated mice transplanted with lung vesicle-modulated marrow have 5 times more WBM-derived type II pneumocytes compared to mice transplanted with unmanipulated WBM. These findings indicate that extracellular vesicle modification may be an important consideration in the development of marrow cell-based cellular therapies. Current studies were performed to determine the specific marrow cell types that LDEV stably modify.
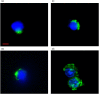
Methods: Murine WBM-derived stem/progenitor cells (Lin-/Sca-1+) and differentiated erythroid cells (Ter119+), granulocytes (Gr-1+) and B cells (CD19+) were cultured with carboxyfluorescein N-succinimidyl ester (CFSE)-labelled LDEV. LDEV+ cells (CFSE+) and LDEV- cells (CFSE-) were separated by flow cytometry and visualized by fluorescence microscopy, analyzed by RT-PCR or placed into long-term secondary culture. In addition, murine Lin-/Sca-1+ cells were cultured with CFSE-labelled LDEV isolated from rats, and RT-PCR analysis was performed on LDEV+ and - cells using species-specific primers for surfactant (rat/mouse hybrid co-cultures).
Results: Stem/progenitor cells and all of the differentiated cell types studied internalized LDEV in culture, but heterogeneously. Expression of a panel of pulmonary epithelial cell genes was higher in LDEV+cells compared to LDEV - cells and elevated expression of these genes persisted in long-term culture. Rat/mouse hybrid co-cultures revealed only mouse-specific surfactant B and C expression in LDEV+ Lin-/Sca-1+cells after 4 weeks of culture, indicating stable de novo gene expression.
Conclusions: LDEV can be internalized by differentiated and more primitive cells residing in the bone marrow in culture and can induce stable de novo pulmonary epithelial cell gene expression in these cells for several weeks after internalization. The gene expression represents a transcriptional activation of the target marrow cells. These studies serve as the basis for determining marrow cell types that can be used for cell-based therapies for processes that injure the pulmonary epithelial surfaces.
Keywords: bone marrow cells; extracellular vesicles; pulmonary epithelial cells.
Figures




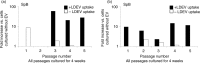
References
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. - PubMed
-
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97. - PubMed
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88. - PubMed
-
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

