Influenza A virus transmission via respiratory aerosols or droplets as it relates to pandemic potential
- PMID: 26385895
- PMCID: PMC5006288
- DOI: 10.1093/femsre/fuv039
Influenza A virus transmission via respiratory aerosols or droplets as it relates to pandemic potential
Abstract
Many respiratory viruses of humans originate from animals. For instance, there are now eight paramyxoviruses, four coronaviruses and four orthomxoviruses that cause recurrent epidemics in humans but were once confined to other hosts. In the last decade, several members of the same virus families have jumped the species barrier from animals to humans. Fortunately, these viruses have not become established in humans, because they lacked the ability of sustained transmission between humans. However, these outbreaks highlighted the lack of understanding of what makes a virus transmissible. In part triggered by the relatively high frequency of occurrence of influenza A virus zoonoses and pandemics, the influenza research community has started to investigate the viral genetic and biological traits that drive virus transmission via aerosols or respiratory droplets between mammals. Here we summarize recent discoveries on the genetic and phenotypic traits required for airborne transmission of zoonotic influenza viruses of subtypes H5, H7 and H9 and pandemic viruses of subtypes H1, H2 and H3. Increased understanding of the determinants and mechanisms of respiratory virus transmission is not only key from a basic scientific perspective, but may also aid in assessing the risks posed by zoonotic viruses to human health, and preparedness for such risks.
Keywords: emerging disease; ferret; guinea pig; host range; pandemic.
© FEMS 2015. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

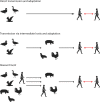
References
-
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–44. - PubMed
-
- Andrewes CH, Glover RE. Spread of infection from the respiratory tract of the ferret. I. Transmission of influenza a virus. Brit J Exp Pathol. 1941;22:91–7.
-
- Artois M, Bicout D, Doctrinal D, et al. Outbreaks of highly pathogenic avian influenza in Europe: the risks associated with wild birds. Rev Sci Tech Oie. 2009;28:69–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

