Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses
- PMID: 26386265
- PMCID: PMC4754969
- DOI: 10.1111/ejn.13081
Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses
Abstract
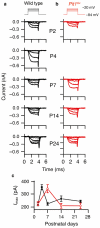
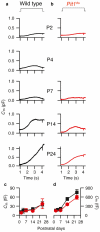
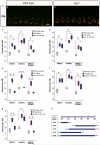
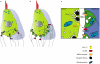
Functional maturation of afferent synaptic connections to inner hair cells (IHCs) involves pruning of excess synapses formed during development, as well as the strengthening and survival of the retained synapses. These events take place during the thyroid hormone (TH)-critical period of cochlear development, which is in the perinatal period for mice and in the third trimester for humans. Here, we used the hypothyroid Snell dwarf mouse (Pit1(dw)) as a model to study the role of TH in afferent type I synaptic refinement and functional maturation. We observed defects in afferent synaptic pruning and delays in calcium channel clustering in the IHCs of Pit1(dw) mice. Nevertheless, calcium currents and capacitance reached near normal levels in Pit1(dw) IHCs by the age of onset of hearing, despite the excess number of retained synapses. We restored normal synaptic pruning in Pit1(dw) IHCs by supplementing with TH from postnatal day (P)3 to P8, establishing this window as being critical for TH action on this process. Afferent terminals of older Pit1(dw) IHCs showed evidence of excitotoxic damage accompanied by a concomitant reduction in the levels of the glial glutamate transporter, GLAST. Our results indicate that a lack of TH during a critical period of inner ear development causes defects in pruning and long-term homeostatic maintenance of afferent synapses.
Keywords: auditory system; calcium current; capacitance measurements; glutamate transporter.
© 2015 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures




Similar articles
-
Thyroid hormone is required for the pruning of afferent type II spiral ganglion neurons in the mouse cochlea.Neuroscience. 2016 Jan 15;312:165-78. doi: 10.1016/j.neuroscience.2015.11.020. Epub 2015 Nov 18. Neuroscience. 2016. PMID: 26592716 Free PMC article.
-
Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels.Neuroscience. 2006 Sep 15;141(4):1849-60. doi: 10.1016/j.neuroscience.2006.05.057. Epub 2006 Jul 10. Neuroscience. 2006. PMID: 16828974
-
The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea.J Neurosci. 2006 Jul 19;26(29):7659-64. doi: 10.1523/JNEUROSCI.1545-06.2006. J Neurosci. 2006. PMID: 16855093 Free PMC article.
-
The auditory hair cell ribbon synapse: from assembly to function.Annu Rev Neurosci. 2012;35:509-28. doi: 10.1146/annurev-neuro-061010-113705. Annu Rev Neurosci. 2012. PMID: 22715884 Review.
-
The afferent signaling complex: Regulation of type I spiral ganglion neuron responses in the auditory periphery.Hear Res. 2016 Jun;336:1-16. doi: 10.1016/j.heares.2016.03.011. Epub 2016 Mar 25. Hear Res. 2016. PMID: 27018296 Review.
Cited by
-
Semaphorin-5B Controls Spiral Ganglion Neuron Branch Refinement during Development.J Neurosci. 2019 Aug 14;39(33):6425-6438. doi: 10.1523/JNEUROSCI.0113-19.2019. Epub 2019 Jun 17. J Neurosci. 2019. PMID: 31209173 Free PMC article.
-
Sensory transduction is required for normal development and maturation of cochlear inner hair cell synapses.Elife. 2021 Nov 4;10:e69433. doi: 10.7554/eLife.69433. Elife. 2021. PMID: 34734805 Free PMC article.
-
Molecular Assembly and Structural Plasticity of Sensory Ribbon Synapses-A Presynaptic Perspective.Int J Mol Sci. 2020 Nov 19;21(22):8758. doi: 10.3390/ijms21228758. Int J Mol Sci. 2020. PMID: 33228215 Free PMC article. Review.
-
Kölliker's organ-supporting cells and cochlear auditory development.Front Mol Neurosci. 2022 Oct 11;15:1031989. doi: 10.3389/fnmol.2022.1031989. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36304996 Free PMC article. Review.
-
Disruption of Glutamate Release and Uptake-Related Protein Expression After Noise-Induced Synaptopathy in the Cochlea.Front Cell Dev Biol. 2021 Aug 4;9:720902. doi: 10.3389/fcell.2021.720902. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422838 Free PMC article.
References
-
- Bernal J. Thyroid hormones and brain development. Vitam. Horm. 2005;71:95–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

