Hydrogel arrays formed via differential wettability patterning enable combinatorial screening of stem cell behavior
- PMID: 26386315
- PMCID: PMC4794413
- DOI: 10.1016/j.actbio.2015.09.019
Hydrogel arrays formed via differential wettability patterning enable combinatorial screening of stem cell behavior
Abstract
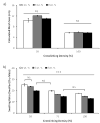
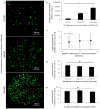
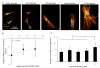
Here, we have developed a novel method for forming hydrogel arrays using surfaces patterned with differential wettability. Our method for benchtop array formation is suitable for enhanced-throughput, combinatorial screening of biochemical and biophysical cues from chemically defined cell culture substrates. We demonstrated the ability to generate these arrays without the need for liquid handling systems and screened the combinatorial effects of substrate stiffness and immobilized cell adhesion peptide concentration on human mesenchymal stem cell (hMSC) behavior during short-term 2-dimensional cell culture. Regardless of substrate stiffness, hMSC initial cell attachment, spreading, and proliferation were linearly correlated with immobilized CRGDS peptide concentration. Increasing substrate stiffness also resulted in increased hMSC initial cell attachment, spreading, and proliferation; however, examination of the combinatorial effects of CRGDS peptide concentration and substrate stiffness revealed potential interplay between these distinct substrate signals. Maximal hMSC proliferation seen on substrates with either high stiffness or high CRGDS peptide concentration suggests that some baseline level of cytoskeletal tension was required for hMSC proliferation on hydrogel substrates and that multiple substrate signals could be engineered to work in synergy to promote mechanosensing and regulate cell behavior.
Statement of significance: Our novel array formation method using surfaces patterned with differential wettability offers the advantages of benchtop array formation for 2-dimensional cell cultures and enhanced-throughput screening without the need for liquid handling systems. Hydrogel arrays formed via our method are suitable for screening the influence of chemical (e.g. cell adhesive ligands) and physical (stiffness, size, shape, and thickness) substrate properties on stem cell behavior. The arrays are also fully compatible with commercially available micro-array add-on systems, which allows for simultaneous control of the insoluble and soluble cell culture environment. This study used hydrogel arrays to demonstrate that synergy between cell adhesion and mechanosensing can be used to regulate hMSC behavior.
Keywords: Cell adhesion; Human mesenchymal stem cell; Mechanosensing; Poly(ethylene glycol); RGD peptide; Thiol-ene.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Microcarriers with Synthetic Hydrogel Surfaces for Stem Cell Expansion.Adv Healthc Mater. 2017 Aug;6(16):10.1002/adhm.201700072. doi: 10.1002/adhm.201700072. Epub 2017 May 16. Adv Healthc Mater. 2017. PMID: 28509413 Free PMC article.
-
Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading.J Biomed Mater Res A. 2010 Jun 1;93(3):1110-23. doi: 10.1002/jbm.a.32601. J Biomed Mater Res A. 2010. PMID: 19768790 Free PMC article.
-
Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays.Tissue Eng Part A. 2009 Feb;15(2):343-53. doi: 10.1089/ten.tea.2008.0096. Tissue Eng Part A. 2009. PMID: 18759676 Free PMC article.
-
How cells sense extracellular matrix stiffness: a material's perspective.Curr Opin Biotechnol. 2013 Oct;24(5):948-53. doi: 10.1016/j.copbio.2013.03.020. Epub 2013 Apr 20. Curr Opin Biotechnol. 2013. PMID: 23611564 Free PMC article. Review.
-
Particle-Based Microarrays of Oligonucleotides and Oligopeptides.Microarrays (Basel). 2014 Oct 28;3(4):245-62. doi: 10.3390/microarrays3040245. Microarrays (Basel). 2014. PMID: 27600347 Free PMC article. Review.
Cited by
-
In situ 3D bioprinting with bioconcrete bioink.Nat Commun. 2022 Jun 23;13(1):3597. doi: 10.1038/s41467-022-30997-y. Nat Commun. 2022. PMID: 35739106 Free PMC article.
-
Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion.Nat Biomed Eng. 2017;1:0096. doi: 10.1038/s41551-017-0096. Epub 2017 Jul 11. Nat Biomed Eng. 2017. PMID: 29104816 Free PMC article.
-
Microcarriers with Synthetic Hydrogel Surfaces for Stem Cell Expansion.Adv Healthc Mater. 2017 Aug;6(16):10.1002/adhm.201700072. doi: 10.1002/adhm.201700072. Epub 2017 May 16. Adv Healthc Mater. 2017. PMID: 28509413 Free PMC article.
-
Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche.Annu Rev Biomed Eng. 2018 Jun 4;20:21-47. doi: 10.1146/annurev-bioeng-062117-120954. Epub 2017 Dec 8. Annu Rev Biomed Eng. 2018. PMID: 29220201 Free PMC article. Review.
-
Biomaterials for Cell Manufacturing.ACS Macro Lett. 2024 Nov 19;13(11):1521-1530. doi: 10.1021/acsmacrolett.4c00634. Epub 2024 Oct 28. ACS Macro Lett. 2024. PMID: 39466845 Free PMC article.
References
-
- Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nature methods. 2011;8:949–55. - PubMed
-
- Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34:8140–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

