Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial
- PMID: 26386540
- PMCID: PMC4888059
- DOI: 10.1016/S0140-6736(15)00239-1
Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial
Abstract
Background: Despite preventive vaccines for oncogenic human papillomaviruses (HPVs), cervical intraepithelial neoplasia (CIN) is common, and current treatments are ablative and can lead to long-term reproductive morbidity. We assessed whether VGX-3100, synthetic plasmids targeting HPV-16 and HPV-18 E6 and E7 proteins, delivered by electroporation, would cause histopathological regression in women with CIN2/3.
Methods: Efficacy, safety, and immunogenicity of VGX-3100 were assessed in CIN2/3 associated with HPV-16 and HPV-18, in a randomised, double-blind, placebo-controlled phase 2b study. Patients from 36 academic and private gynaecology practices in seven countries were randomised (3:1) to receive 6 mg VGX-3100 or placebo (1 mL), given intramuscularly at 0, 4, and 12 weeks. Randomisation was stratified by age (<25 vs ≥25 years) and CIN2 versus CIN3 by computer-generated allocation sequence (block size 4). Funder and site personnel, participants, and pathologists were masked to treatment. The primary efficacy endpoint was regression to CIN1 or normal pathology 36 weeks after the first dose. Per-protocol and modified intention-to-treat analyses were based on patients receiving three doses without protocol violations, and on patients receiving at least one dose, respectively. The safety population included all patients who received at least one dose. The trial is registered at ClinicalTrials.gov (number NCT01304524) and EudraCT (number 2012-001334-33).
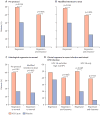
Findings: Between Oct 19, 2011, and July 30, 2013, 167 patients received either VGX-3100 (n=125) or placebo (n=42). In the per-protocol analysis 53 (49·5%) of 107 VGX-3100 recipients and 11 (30·6%) of 36 placebo recipients had histopathological regression (percentage point difference 19·0 [95% CI 1·4-36·6]; p=0·034). In the modified intention-to-treat analysis 55 (48·2%) of 114 VGX-3100 recipients and 12 (30·0%) of 40 placebo recipients had histopathological regression (percentage point difference 18·2 [95% CI 1·3-34·4]; p=0·034). Injection-site reactions occurred in most patients, but only erythema was significantly more common in the VGX-3100 group (98/125, 78·4%) than in the placebo group (24/42, 57·1%; percentage point difference 21·3 [95% CI 5·3-37·8]; p=0·007).
Interpretation: VGX-3100 is the first therapeutic vaccine to show efficacy against CIN2/3 associated with HPV-16 and HPV-18. VGX-3100 could present a non-surgical therapeutic option for CIN2/3, changing the treatment outlook for this common disease.
Funding: Inovio Pharmaceuticals.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Towards therapeutic vaccination against cervical precancer?Lancet. 2015 Nov 21;386(10008):2036-2038. doi: 10.1016/S0140-6736(15)00240-8. Epub 2015 Sep 17. Lancet. 2015. PMID: 26386539 No abstract available.
-
Therapeutic HPV vaccine holds promise.Nat Rev Clin Oncol. 2015 Dec;12(12):686. doi: 10.1038/nrclinonc.2015.180. Epub 2015 Oct 13. Nat Rev Clin Oncol. 2015. PMID: 26462124 No abstract available.
References
-
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. - PubMed
-
- Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(suppl):S20–26. - PubMed
-
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–28. - PubMed
-
- Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

