RNA interference machinery-mediated gene regulation in mouse adult neural stem cells
- PMID: 26386671
- PMCID: PMC4575781
- DOI: 10.1186/s12868-015-0198-7
RNA interference machinery-mediated gene regulation in mouse adult neural stem cells
Abstract
Background: Neurogenesis in the brain of adult mammals occurs throughout life in two locations: the subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus in the hippocampus. RNA interference mechanisms have emerged as critical regulators of neuronal differentiation. However, to date, little is known about its function in adult neurogenesis.
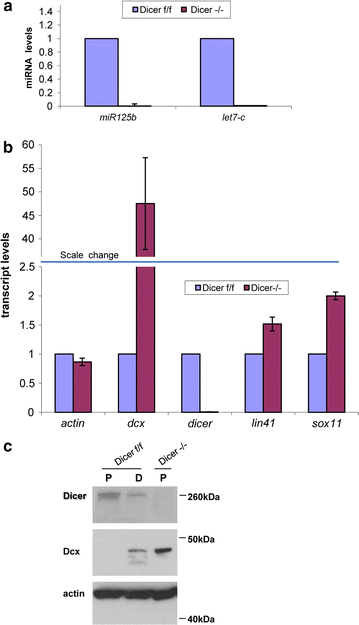
Results: Here we show that the RNA interference machinery regulates Doublecortin levels and is associated with chromatin in differentiating adult neural progenitors. Deletion of Dicer causes abnormal higher levels of Doublecortin. The microRNA pathway plays an important role in Doublecortin regulation. In particular miRNA-128 overexpression can reduce Doublecortin levels in differentiating adult neural progenitors.
Conclusions: We conclude that the RNA interference components play an important role, even through chromatin association, in regulating neuron-specific gene expression programs.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

