Beyond spectral tuning: human cone visual pigments adopt different transient conformations for chromophore regeneration
- PMID: 26387074
- PMCID: PMC11108329
- DOI: 10.1007/s00018-015-2043-7
Beyond spectral tuning: human cone visual pigments adopt different transient conformations for chromophore regeneration
Abstract
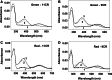
Human red and green visual pigments are seven transmembrane receptors of cone photoreceptor cells of the retina that mediate color vision. These pigments share a very high degree of homology and have been assumed to feature analogous structural and functional properties. We report on a different regeneration mechanism among red and green cone opsins with retinal analogs using UV-Vis/fluorescence spectroscopic analyses, molecular modeling and site-directed mutagenesis. We find that photoactivated green cone opsin adopts a transient conformation which regenerates via an unprotonated Schiff base linkage with its natural chromophore, whereas red cone opsin forms a typical protonated Schiff base. The chromophore regeneration kinetics is consistent with a secondary retinal uptake by the cone pigments. Overall, our findings reveal, for the first time, structural differences in the photoactivated conformation between red and green cone pigments that may be linked to their molecular evolution, and support the proposal of secondary retinal binding to visual pigments, in addition to binding to the canonical primary site, which may serve as a regulatory mechanism of dark adaptation in the phototransduction process.
Keywords: Color vision; G-protein coupled receptors; Ligand binding; Retinal; Visual phototransduction.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

