Hyperspectral Microscopy of Near-Infrared Fluorescence Enables 17-Chirality Carbon Nanotube Imaging
- PMID: 26387482
- PMCID: PMC4585673
- DOI: 10.1038/srep14167
Hyperspectral Microscopy of Near-Infrared Fluorescence Enables 17-Chirality Carbon Nanotube Imaging
Abstract
The intrinsic near-infrared photoluminescence (fluorescence) of single-walled carbon nanotubes exhibits unique photostability, narrow bandwidth, penetration through biological media, environmental sensitivity, and both chromatic variety and range. Biomedical applications exploiting this large family of fluorophores will require the spectral and spatial resolution of individual (n,m) nanotube species' fluorescence and its modulation within live cells and tissues, which is not possible with current microscopy methods. We present a wide-field hyperspectral approach to spatially delineate and spectroscopically measure single nanotube fluorescence in living systems. This approach resolved up to 17 distinct (n,m) species (chiralities) with single nanotube spatial resolution in live mammalian cells, murine tissues ex vivo, and zebrafish endothelium in vivo. We anticipate that this approach will facilitate multiplexed nanotube imaging in biomedical applications while enabling deep-tissue optical penetration, and single-molecule resolution in vivo.
Conflict of interest statement
S.B.-O. is founder of, and S.M. and M.V. are employees of Photon Etc., a company that manufactures hyperspectral imaging technology.
Figures

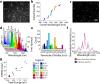
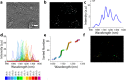
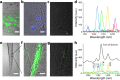
References
-
- Cheong W.-F., Prahl S. A. & Welch A. J. A review of the optical properties of biological tissues. IEEE journal of quantum electronics 26, 2166–2185 (1990).
-
- Lichtman J. W. & Conchello J.-A. Fluorescence microscopy. Nat Meth 2, 910–919 (2005). - PubMed
-
- Cherukuri P., Bachilo S. M., Litovsky S. H. & Weisman R. B. Near-Infrared Fluorescence Microscopy of Single-Walled Carbon Nanotubes in Phagocytic Cells. J. Am. Chem. Soc. 126, 15638–15639 (2004). - PubMed
-
- O’Connell M. J. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593–596 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

