SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore
- PMID: 26387735
- PMCID: PMC4592475
- DOI: 10.1016/j.molcel.2015.08.009
SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore
Abstract
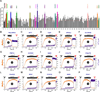
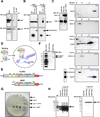
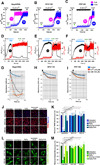
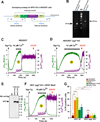
Mitochondrial permeability transition is a phenomenon in which the mitochondrial permeability transition pore (PTP) abruptly opens, resulting in mitochondrial membrane potential (ΔΨm) dissipation, loss of ATP production, and cell death. Several genetic candidates have been proposed to form the PTP complex, however, the core component is unknown. We identified a necessary and conserved role for spastic paraplegia 7 (SPG7) in Ca(2+)- and ROS-induced PTP opening using RNAi-based screening. Loss of SPG7 resulted in higher mitochondrial Ca(2+) retention, similar to cyclophilin D (CypD, PPIF) knockdown with sustained ΔΨm during both Ca(2+) and ROS stress. Biochemical analyses revealed that the PTP is a heterooligomeric complex composed of VDAC, SPG7, and CypD. Silencing or disruption of SPG7-CypD binding prevented Ca(2+)- and ROS-induced ΔΨm depolarization and cell death. This study identifies an ubiquitously expressed IMM integral protein, SPG7, as a core component of the PTP at the OMM and IMM contact site.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. - PMC - PubMed
-
- Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem. 2001;276:1954–1960. - PubMed
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

