Preexisting smooth muscle cells contribute to neointimal cell repopulation at an incidence varying widely among individual lesions
- PMID: 26387788
- PMCID: PMC4707076
- DOI: 10.1016/j.surg.2015.08.015
Preexisting smooth muscle cells contribute to neointimal cell repopulation at an incidence varying widely among individual lesions
Abstract
Background: With the diverse origin of neointimal cells, previous studies have documented differences of neointimal cell lineage composition across models, but the animal-to-animal difference has not attracted much attention, although the cellular heterogeneity may impact neointimal growth and its response to therapeutic interventions.
Methods: R26R(+);Myh11-CreER(+), and R26R(+);Scl-CreER(+) mice were used to attach LacZ tags to the preexisting smooth muscle cells (SMCs) and endothelial cells (ECs), respectively. Neointimal lesions were created via complete ligation of the common carotid artery (CCA) and transluminal injury to the femoral artery (FA).
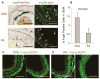
Results: LacZ-tagged SMCs were physically relocated from media to neointima and changed to a dedifferentiated phenotype in both CCA and FA lesions. The content of SMCs in the neointimal tissue, however, varied widely among specimens, ranging from 5 to 70% and 0 to 85%, with an average at low levels of 27% and 29% in CCA (n = 15) and FA (n = 15) lesions, respectively. Bone marrow cells, although able to home to the injured arteries, did not differentiate fully into SMCs after either type of injury. Preexisting ECs were located in the subendothelial region and produced mesenchymal marker α-actin, indicating endothelial-mesenchymal transition (EndoMT); however, EC-derived cells represented only 7% and 3% of the total neointimal cell pool of CCA (n = 7) and FA (n = 7) lesions, respectively. ECs located on the luminal surface exhibited little evidence of EndoMT.
Conclusion: Neointimal hyperplasia proceeds with a wide range of variation in its cellular composition between individual lesions. Relative to ECs, SMCs are major contributors to the lesion-to-lesion heterogeneity in neointimal cell lineage composition.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Imai H, Lee KJ, Lee SK, Lee KT, O’Neal RM, Thomas WA. Ultrastructural features of aortic cells in mitosis in control and cholesterol-fed swine. Lab Invest. 1970;23:401–415. - PubMed
-
- Lee KT, Thomas WA, Florentin RA, Reiner JM, Lee WM. Evidence for a polyclonal origin and proliferative heterogeneity of atherosclerotic lesions induced by dietary cholesterol in young swine. Ann N Y Acad Sci. 1976;275:336–347. - PubMed
-
- Clowes AW, Clowes MM, Reidy MA. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986;54:295–303. - PubMed
-
- Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. - PubMed
-
- Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13–e20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

