The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders
- PMID: 26388713
- PMCID: PMC4555040
- DOI: 10.3389/fnins.2015.00296
The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders
Abstract
The cerebellum has been repeatedly implicated in gene expression, rodent model and post-mortem studies of autism spectrum disorder (ASD). How cellular and molecular anomalies of the cerebellum relate to clinical manifestations of ASD remains unclear. Separate circuits of the cerebellum control different sensorimotor behaviors, such as maintaining balance, walking, making eye movements, reaching, and grasping. Each of these behaviors has been found to be impaired in ASD, suggesting that multiple distinct circuits of the cerebellum may be involved in the pathogenesis of patients' sensorimotor impairments. We will review evidence that the development of these circuits is disrupted in individuals with ASD and that their study may help elucidate the pathophysiology of sensorimotor deficits and core symptoms of the disorder. Preclinical studies of monogenetic conditions associated with ASD also have identified selective defects of the cerebellum and documented behavioral rescues when the cerebellum is targeted. Based on these findings, we propose that cerebellar circuits may prove to be promising targets for therapeutic development aimed at rescuing sensorimotor and other clinical symptoms of different forms of ASD.
Keywords: autism spectrum disorder; cerebellum; gait; genetics; oculomotor; pathophysiology; precision grip; sensorimotor.
Figures

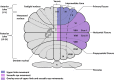

References
-
- (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian fragile X consortium. Cell 78, 23–33. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

