Prenatal Hypoxia-Ischemia Induces Abnormalities in CA3 Microstructure, Potassium Chloride Co-Transporter 2 Expression and Inhibitory Tone
- PMID: 26388734
- PMCID: PMC4558523
- DOI: 10.3389/fncel.2015.00347
Prenatal Hypoxia-Ischemia Induces Abnormalities in CA3 Microstructure, Potassium Chloride Co-Transporter 2 Expression and Inhibitory Tone
Abstract
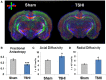
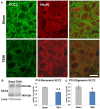
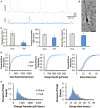
Infants who suffer perinatal brain injury, including those with encephalopathy of prematurity, are prone to chronic neurological deficits, including epilepsy, cognitive impairment, and behavioral problems, such as anxiety, inattention, and poor social interaction. These deficits, especially in combination, pose the greatest hindrance to these children becoming independent adults. Cerebral function depends on adequate development of essential inhibitory neural circuits and the appropriate amount of excitation and inhibition at specific stages of maturation. Early neuronal synaptic responses to γ-amino butyric acid (GABA) are initially excitatory. During the early postnatal period, GABAAR responses switch to inhibitory with the upregulation of potassium-chloride co-transporter KCC2. With extrusion of chloride by KCC2, the Cl(-) reversal potential shifts and GABA and glycine responses become inhibitory. We hypothesized that prenatal hypoxic-ischemic brain injury chronically impairs the developmental upregulation of KCC2 that is essential for cerebral circuit formation. Following late gestation hypoxia-ischemia (HI), diffusion tensor imaging in juvenile rats shows poor microstructural integrity in the hippocampal CA3 subfield, with reduced fractional anisotropy and elevated radial diffusivity. The loss of microstructure correlates with early reduced KCC2 expression on NeuN-positive pyramidal neurons, and decreased monomeric and oligomeric KCC2 protein expression in the CA3 subfield. Together with decreased inhibitory post-synaptic currents during a critical window of development, we document for the first time that prenatal transient systemic HI in rats impairs hippocampal CA3 inhibitory tone. Failure of timely development of inhibitory tone likely contributes to a lower seizure threshold and impaired cognitive function in children who suffer perinatal brain injury.
Keywords: KCC2; epilepsy; hippocampus; microstructure; prematurity; seizure.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

