Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages
- PMID: 26388857
- PMCID: PMC4558532
- DOI: 10.3389/fmicb.2015.00915
Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages
Abstract
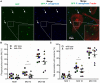
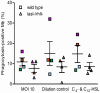
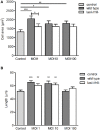
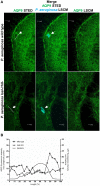
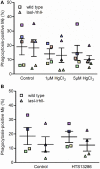
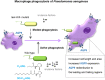
Pseudomonas aeruginosa controls production of its multiple virulence factors and biofilm development via the quorum sensing (QS) system. QS signals also interact with and affect the behavior of eukaryotic cells. Host water homeostasis and aquaporins (AQP) are essential during pathological conditions since they interfere with the cell cytoskeleton and signaling, and hereby affect cell morphology and functions. We investigated the contribution of P. aeruginosa QS genes lasI/rhlI to phagocytosis, cell morphology, AQP9 expression, and distribution in human macrophages, using immunoblotting, confocal, and nanoscale imaging. Wild type P. aeruginosa with a functional QS system was a more attractive prey for macrophages than the lasI/rhlI mutant lacking the production of QS molecules, 3O-C12-HSL, and C4-HSL, and associated virulence factors. The P. aeruginosa infections resulted in elevated AQP9 expression and relocalization to the leading and trailing regions in macrophages, increased cell area and length; bacteria with a functional QS system lasI/rhlI achieved stronger responses. We present evidence for a new role of water fluxes via AQP9 during bacteria-macrophage interaction and for the QS system as an important stimulus in this process. These novel events in the interplay between P. aeruginosa and macrophages may influence on the outcome of infection, inflammation, and development of disease.
Keywords: N-acylhomoserine lactone; aquaporin; host-bacteria relationship; innate immunity; macrophage; quorum sensing; water homeostasis.
Figures


Similar articles
-
Pseudomonas aeruginosa N-3-oxo-dodecanoyl-homoserine Lactone Elicits Changes in Cell Volume, Morphology, and AQP9 Characteristics in Macrophages.Front Cell Infect Microbiol. 2016 Mar 24;6:32. doi: 10.3389/fcimb.2016.00032. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27047801 Free PMC article.
-
Mutation of Pseudomonas aeruginosa lasI/rhlI diminishes its cytotoxicity, oxidative stress, inflammation, and apoptosis on THP-1 macrophages.Microbiol Spectr. 2024 Oct 3;12(10):e0414623. doi: 10.1128/spectrum.04146-23. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162513 Free PMC article.
-
Quorum sensing and virulence of Pseudomonas aeruginosa during urinary tract infections.J Infect Dev Ctries. 2012 Jun 15;6(6):501-7. doi: 10.3855/jidc.2543. J Infect Dev Ctries. 2012. PMID: 22706193
-
[Quorum sensing: a new clinical target for Pseudomonas aeruginosa?].Med Mal Infect. 2006 Jul;36(7):349-57. doi: 10.1016/j.medmal.2006.01.008. Epub 2006 May 2. Med Mal Infect. 2006. PMID: 16631332 Review. French.
-
Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article.Immunol Lett. 2017 Oct;190:1-6. doi: 10.1016/j.imlet.2017.07.002. Epub 2017 Jul 8. Immunol Lett. 2017. PMID: 28698104 Review.
Cited by
-
The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker.Biomolecules. 2022 Jun 27;12(7):897. doi: 10.3390/biom12070897. Biomolecules. 2022. PMID: 35883453 Free PMC article. Review.
-
The multifaceted role of aquaporins in physiological cell migration.Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C208-C223. doi: 10.1152/ajpcell.00502.2022. Epub 2023 May 29. Am J Physiol Cell Physiol. 2023. PMID: 37246634 Free PMC article. Review.
-
Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity.Front Immunol. 2020 Aug 28;11:1827. doi: 10.3389/fimmu.2020.01827. eCollection 2020. Front Immunol. 2020. PMID: 32983093 Free PMC article. Review.
-
Battle royale: Immune response on biofilms - host-pathogen interactions.Curr Res Immunol. 2023 Mar 28;4:100057. doi: 10.1016/j.crimmu.2023.100057. eCollection 2023. Curr Res Immunol. 2023. PMID: 37025390 Free PMC article. Review.
-
Ablation of Aquaporin-9 Ameliorates the Systemic Inflammatory Response of LPS-Induced Endotoxic Shock in Mouse.Cells. 2021 Feb 18;10(2):435. doi: 10.3390/cells10020435. Cells. 2021. PMID: 33670755 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

