Triple Aryne-Tetrazine Reaction Enabling Rapid Access to a New Class of Polyaromatic Heterocycles
- PMID: 26388984
- PMCID: PMC4570739
- DOI: 10.1039/C5SC01726B
Triple Aryne-Tetrazine Reaction Enabling Rapid Access to a New Class of Polyaromatic Heterocycles
Abstract
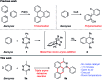
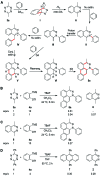
One of the most challenging goals of modern synthetic chemistry is to develop multi-step reactions for rapid and efficient access to complex molecules. We report a triple aryne-tetrazine reaction that enables rapid access to a new class of polyaromatic heterocycles. This new reaction, which couples diverse reactivity modes between simple aryne and tetrazine starting materials, proceeds in a single operation and takes less than 5 minutes in air with no metal catalyst.
Figures


References
-
- Rarig R.-A. F., Tran M. N., Chenoweth D. M. J. Am. Chem. Soc. 2013;135:9213–9219. - PubMed
-
- Tambar U. K., Stoltz B. M. J. Am. Chem. Soc. 2005;127:5340–5341. - PubMed
-
- Gilmore C. D., Allan K. M., Stoltz B. M. J. Am. Chem. Soc. 2008;130:1558–1559. - PubMed
-
- Tadross P. M., Gilmore C. D., Bugga P., Virgil S. C., Stoltz B. M. Org. Lett. 2010;12:1224–1227. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

