A Review: Molecular Aberrations within Hippo Signaling in Bone and Soft-Tissue Sarcomas
- PMID: 26389076
- PMCID: PMC4557106
- DOI: 10.3389/fonc.2015.00190
A Review: Molecular Aberrations within Hippo Signaling in Bone and Soft-Tissue Sarcomas
Abstract
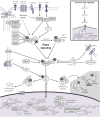
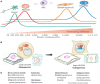
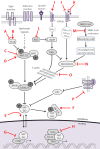
The Hippo signaling pathway is an evolutionarily conserved developmental network vital for the regulation of organ size, tissue homeostasis, repair and regeneration, and cell fate. The Hippo pathway has also been shown to have tumor suppressor properties. Hippo transduction involves a series of kinases and scaffolding proteins that are intricately connected to proteins in developmental cascades and in the tissue microenvironment. This network governs the downstream Hippo transcriptional co-activators, YAP and TAZ, which bind to and activate the output of TEADs, as well as other transcription factors responsible for cellular proliferation, self-renewal, differentiation, and survival. Surprisingly, there are few oncogenic mutations within the core components of the Hippo pathway. Instead, dysregulated Hippo signaling is a versatile accomplice to commonly mutated cancer pathways. For example, YAP and TAZ can be activated by oncogenic signaling from other pathways, or serve as co-activators for classical oncogenes. Emerging evidence suggests that Hippo signaling couples cell density and cytoskeletal structural changes to morphogenic signals and conveys a mesenchymal phenotype. While much of Hippo biology has been described in epithelial cell systems, it is clear that dysregulated Hippo signaling also contributes to malignancies of mesenchymal origin. This review will summarize the known molecular alterations within the Hippo pathway in sarcomas and highlight how several pharmacologic compounds have shown activity in modulating Hippo components, providing proof-of-principle that Hippo signaling may be harnessed for therapeutic application in sarcomas.
Keywords: Ewing sarcoma; Hippo; mesenchymal; osteosarcoma; pediatric cancers; rhabdomyosarcoma; sarcoma; targeted therapy.
Figures
Similar articles
-
TEAD1 mediates the oncogenic activities of Hippo-YAP1 signaling in osteosarcoma.Biochem Biophys Res Commun. 2017 Jun 24;488(2):297-302. doi: 10.1016/j.bbrc.2017.05.032. Epub 2017 May 5. Biochem Biophys Res Commun. 2017. PMID: 28483529
-
Targeting the Hippo Signaling Pathway for Tissue Regeneration and Cancer Therapy.Genes (Basel). 2016 Aug 30;7(9):55. doi: 10.3390/genes7090055. Genes (Basel). 2016. PMID: 27589805 Free PMC article. Review.
-
Genetic Alterations and Deregulation of Hippo Pathway as a Pathogenetic Mechanism in Bone and Soft Tissue Sarcoma.Cancers (Basel). 2022 Dec 15;14(24):6211. doi: 10.3390/cancers14246211. Cancers (Basel). 2022. PMID: 36551696 Free PMC article. Review.
-
Molecular mechanisms of the mammalian Hippo signaling pathway.Yi Chuan. 2017 Jul 20;39(7):546-567. doi: 10.16288/j.yczz.17-094. Yi Chuan. 2017. PMID: 28757470 Review.
-
The Hippo signaling pathway provides novel anti-cancer drug targets.Oncotarget. 2017 Feb 28;8(9):16084-16098. doi: 10.18632/oncotarget.14306. Oncotarget. 2017. PMID: 28035075 Free PMC article. Review.
Cited by
-
Melatonin Regulates Cisplatin Resistance and Glucose Metabolism Through Hippo Signaling in Hepatocellular Carcinoma Cells.Cancer Manag Res. 2020 Mar 12;12:1863-1874. doi: 10.2147/CMAR.S230466. eCollection 2020. Cancer Manag Res. 2020. PMID: 32210629 Free PMC article.
-
Effect of cytostatic proline rich polypeptide-1 on tumor suppressors of inflammation pathway signaling in chondrosarcoma.Mol Clin Oncol. 2016 Nov;5(5):618-624. doi: 10.3892/mco.2016.1010. Epub 2016 Sep 2. Mol Clin Oncol. 2016. PMID: 27900099 Free PMC article.
-
Epithelioid Hemangioendothelioma as a Model of YAP/TAZ-Driven Cancer: Insights from a Rare Fusion Sarcoma.Cancers (Basel). 2018 Jul 10;10(7):229. doi: 10.3390/cancers10070229. Cancers (Basel). 2018. PMID: 29996478 Free PMC article. Review.
-
Genetic Screen in a Preclinical Model of Sarcoma Development Defines Drivers and Therapeutic Vulnerabilities.Clin Cancer Res. 2024 Nov 1;30(21):4957-4973. doi: 10.1158/1078-0432.CCR-24-1238. Clin Cancer Res. 2024. PMID: 39177582 Free PMC article.
-
YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells.Oncogenesis. 2021 Jan 8;10(1):2. doi: 10.1038/s41389-020-00294-8. Oncogenesis. 2021. PMID: 33419969 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

