Outcome after Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology
- PMID: 26389799
- PMCID: PMC4577091
- DOI: 10.1371/journal.pntd.0003964
Outcome after Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology
Abstract
Background/aims: Benzimidazoles are efficacious for treating non-resectable alveolar echinococcosis (AE), but their long-term parasitocidal (curative) effect is disputed. In this study, we prospectively analyzed the potential parasitocidal effect of benzimidazoles and whether normalization of FDG-PET/CT scans and anti-Emll/3-10-antibody levels could act as reliable "in vivo" parameters of AE-inactivation permitting to abrogate chemotherapy with a low risk for AE-recurrence.
Method: This prospective study included 34 patients with non-resectable AE subdivided into group A (n = 11), followed-up after diagnosis and begin of chemotherapy at months 6, 12 and 24, and group B (n = 23) with a medium duration of chemotherapy of 10 (range 2-25) years. All patients were assessed by FDG-PET/CT examinations and anti-EmII/3-10 serology. Chemotherapy was abrogated in patients with normalization of FDG-PET/CT and serum anti-EmII/3-10 levels. These patients were closely followed-up for AE recurrence. Endpoint (parasitocidal efficacy) was defined by the absence of AE-recurrence >24 months after stopping treatment.
Results: Normalization of FDG-PET/CT scan and anti-EmII/3-10 levels occurred in 11 of 34 patients (32%). After abrogation of chemotherapy in these 11 patients, there was no evidence of AE-recurrence within a median of 70.5 (range 16-82) months. However, the patients' immunocompetence appears pivotal for the described long-term parasitocidal effect of benzimidazoles.
Conclusions: The combination of negative FDG-PET/CT-scans and anti-EmII/3-10 antibody levels seem to be reliable parameters for assessing in vivo AE-larval inactivity after long-term benzimidazole chemotherapy.
Trial registration: clinicaltrials.gov: NCT00658294.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
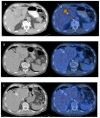

References
-
- Ammann RW, Eckert J (1996) Cestodes. Echinococcus. Gastroenterol Clin North Am 25: 655–689. - PubMed
-
- Eckert J, Deplazes P, Kern P (2011) Alveolar echinococcosis (Echinococcus multilocularis) and neotropical forms of echinococcosis (Echinococcus vogeli and Echinococcus oligarthrus),. In: Palmer SR, Soulsby L, P.R. T, Brown DWG, editors. Oxford Textbook of Zoonoses Biology, Clinical Practice, and Public Health Control: Oxford University Press; pp. 669–699.
-
- Pawlowski Z, Eckert J, Vuitton DA, Ammann R, Kern P (2001) Echinococcosis in humans: clinical aspects, diagnosis and treatment In: Eckert J, Gemmel M, Meslin F, Pawlowski Z, editors. WHO/OIE Manual of Echinococcosis in humans and animals. Paris: WHO organisation for animal health and WHO; pp. 20–71.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

