Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep
- PMID: 26389842
- PMCID: PMC4625581
- DOI: 10.1038/nn.4119
Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep
Abstract
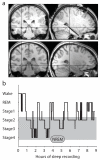
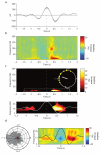
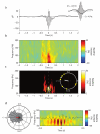
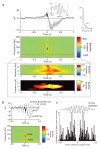
During systems-level consolidation, mnemonic representations initially reliant on the hippocampus are thought to migrate to neocortical sites for more permanent storage, with an eminent role of sleep for facilitating this information transfer. Mechanistically, consolidation processes have been hypothesized to rely on systematic interactions between the three cardinal neuronal oscillations characterizing non-rapid eye movement (NREM) sleep. Under global control of de- and hyperpolarizing slow oscillations (SOs), sleep spindles may cluster hippocampal ripples for a precisely timed transfer of local information to the neocortex. We used direct intracranial electroencephalogram recordings from human epilepsy patients during natural sleep to test the assumption that SOs, spindles and ripples are functionally coupled in the hippocampus. Employing cross-frequency phase-amplitude coupling analyses, we found that spindles were modulated by the up-state of SOs. Notably, spindles were found to in turn cluster ripples in their troughs, providing fine-tuned temporal frames for the hypothesized transfer of hippocampal memory traces.
Figures
References
-
- Born J, Rasch B, Gais S. Sleep to remember. The Neuroscientist. 2006;12:410–424. - PubMed
-
- Buzsáki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. - PubMed
-
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological review. 1995;102:419. - PubMed
-
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11:114–126. - PubMed
Methods references
-
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007.
-
- Berens P. CircStat: a Matlab Toolbox for Circular Statistics. Journal of Statistical Software. 2009;31
-
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. Journal of neuroscience methods. 2007;164:177–190. - PubMed
-
- Mormann F, et al. Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

