Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine's Tool Box
- PMID: 26389876
- PMCID: PMC6331900
- DOI: 10.3390/molecules200916852
Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine's Tool Box
Abstract
The majority of heterocycle compounds and typically common heterocycle fragments present in most pharmaceuticals currently marketed, alongside with their intrinsic versatility and unique physicochemical properties, have poised them as true cornerstones of medicinal chemistry. Apart from the already marketed drugs, there are many other being investigated for their promising activity against several malignancies. In particular, anticancer research has been capitalizing on the intrinsic versatility and dynamic core scaffold of these compounds. Nevertheless, as for any other promising anticancer drugs, heterocyclic compounds do not come without shortcomings. In this review, we provide for a concise overview of heterocyclic active compounds and families and their main applications in medicine. We shall focus on those suitable for cancer therapy while simultaneously addressing main biochemical modes of action, biological targets, structure-activity relationships as well as intrinsic limitation issues in the use of these compounds. Finally, considering the advent of nanotechnology for effective selective targeting of drugs, we shall discuss fundamental aspects and considerations on nanovectorization of such compounds that may improve pharmacokinetic/pharmacodynamic properties of heterocycles.
Keywords: cancer therapy; drug delivery; heterocyclic compounds; nanomedicine; oxygen and nitrogen-based heterocycles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
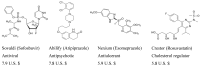
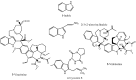

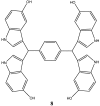
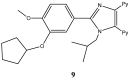




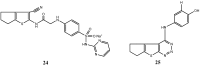
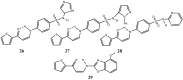
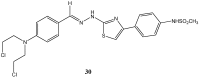
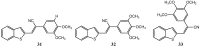

References
-
- IUPAC Gold Book—Heterocyclic Compounds. [(accessed on 26 May 2015)]. Available online: http://goldbook.iupac.org/H02798.html.
-
- Gomtsyan A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012;48:7–10. doi: 10.1007/s10593-012-0960-z. - DOI
-
- Dua R., Shrivastava S., Sonwane S.K., Srivastava S.K. Pharmacological Significance of Synthetic Heterocycles Scaffold : A Review. Adv. Biol. Res. (Rennes). 2011;5:120–144.
-
- Eicher T., Hauptmann S., Speicher A., editors. The Chemistry of Heterocycles: Structure, Reactions, Synthesis, and Applications. 3rd ed. Wiley-VCH; Weinheim, Germany: 2012. The Structure of Heterocyclic Compounds; pp. 1–4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

