Photosynthetic Pigments in Diatoms
- PMID: 26389924
- PMCID: PMC4584358
- DOI: 10.3390/md13095847
Photosynthetic Pigments in Diatoms
Abstract
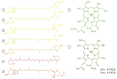
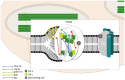
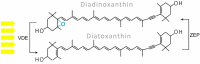
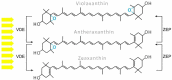
Photosynthetic pigments are bioactive compounds of great importance for the food, cosmetic, and pharmaceutical industries. They are not only responsible for capturing solar energy to carry out photosynthesis, but also play a role in photoprotective processes and display antioxidant activity, all of which contribute to effective biomass and oxygen production. Diatoms are organisms of a distinct pigment composition, substantially different from that present in plants. Apart from light-harvesting pigments such as chlorophyll a, chlorophyll c, and fucoxanthin, there is a group of photoprotective carotenoids which includes β-carotene and the xanthophylls, diatoxanthin, diadinoxanthin, violaxanthin, antheraxanthin, and zeaxanthin, which are engaged in the xanthophyll cycle. Additionally, some intermediate products of biosynthetic pathways have been identified in diatoms as well as unusual pigments, e.g., marennine. Marine algae have become widely recognized as a source of unique bioactive compounds for potential industrial, pharmaceutical, and medical applications. In this review, we summarize current knowledge on diatom photosynthetic pigments complemented by some new insights regarding their physico-chemical properties, biological role, and biosynthetic pathways, as well as the regulation of pigment level in the cell, methods of purification, and significance in industries.
Keywords: bioactive compounds; biosynthesis pathway; diatoms; photoprotection; photosynthesis; pigments.
Figures

Similar articles
-
Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle.Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8784-9. doi: 10.1073/pnas.96.15.8784. Proc Natl Acad Sci U S A. 1999. PMID: 10411953 Free PMC article.
-
Xanthophyll synthesis in diatoms: quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model.Planta. 2001 Feb;212(3):382-91. doi: 10.1007/s004250000403. Planta. 2001. PMID: 11289603
-
The effect of different light regimes on pigments in Coscinodiscus granii.Photosynth Res. 2019 Jun;140(3):301-310. doi: 10.1007/s11120-018-0608-7. Epub 2018 Nov 26. Photosynth Res. 2019. PMID: 30478709
-
Marennine, promising blue pigments from a widespread Haslea diatom species complex.Mar Drugs. 2014 May 28;12(6):3161-89. doi: 10.3390/md12063161. Mar Drugs. 2014. PMID: 24879542 Free PMC article. Review.
-
Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence.J Photochem Photobiol B. 1996 Oct;36(1):3-15. doi: 10.1016/S1011-1344(96)07397-6. J Photochem Photobiol B. 1996. PMID: 8988608 Review.
Cited by
-
Regulation of Carbon Metabolism by Environmental Conditions: A Perspective From Diatoms and Other Chromalveolates.Front Plant Sci. 2020 Jul 16;11:1033. doi: 10.3389/fpls.2020.01033. eCollection 2020. Front Plant Sci. 2020. PMID: 32765548 Free PMC article. Review.
-
Symbiodiniaceae photophysiology and stress resilience is enhanced by microbial associations.Sci Rep. 2023 Nov 25;13(1):20724. doi: 10.1038/s41598-023-48020-9. Sci Rep. 2023. PMID: 38007500 Free PMC article.
-
Enhanced pigment content estimation using the Gauss-peak spectra method with thin-layer chromatography for a novel source of natural colorants.PLoS One. 2021 May 12;16(5):e0251491. doi: 10.1371/journal.pone.0251491. eCollection 2021. PLoS One. 2021. PMID: 33979411 Free PMC article.
-
Overexpression of PtVDL1 in Phaeodactylum tricornutum Increases Fucoxanthin Content under Red Light.J Microbiol Biotechnol. 2024 Jan 28;34(1):198-206. doi: 10.4014/jmb.2309.09018. Epub 2023 Oct 20. J Microbiol Biotechnol. 2024. PMID: 37957112 Free PMC article.
-
Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects In Vitro and In Vivo (Zebrafish Model).Mar Drugs. 2021 Apr 22;19(5):235. doi: 10.3390/md19050235. Mar Drugs. 2021. PMID: 33922234 Free PMC article.
References
-
- Martin-Jézéquel V., Hildebrand M., Brzezinski M.A. Silicon metabolisim in diatoms: Implications for growth. J. Phycol. 2000;36:821–840. doi: 10.1046/j.1529-8817.2000.00019.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

