Alterations of Nuclear Architecture and Epigenetic Signatures during African Swine Fever Virus Infection
- PMID: 26389938
- PMCID: PMC4584302
- DOI: 10.3390/v7092858
Alterations of Nuclear Architecture and Epigenetic Signatures during African Swine Fever Virus Infection
Abstract
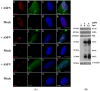
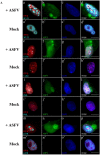
Viral interactions with host nucleus have been thoroughly studied, clarifying molecular mechanisms and providing new antiviral targets. Considering that African swine fever virus (ASFV) intranuclear phase of infection is poorly understood, viral interplay with subnuclear domains and chromatin architecture were addressed. Nuclear speckles, Cajal bodies, and promyelocytic leukaemia nuclear bodies (PML-NBs) were evaluated by immunofluorescence microscopy and Western blot. Further, efficient PML protein knockdown by shRNA lentiviral transduction was used to determine PML-NBs relevance during infection. Nuclear distribution of different histone H3 methylation marks at lysine's 9, 27 and 36, heterochromatin protein 1 isoforms (HP1α, HPβ and HPγ) and several histone deacetylases (HDACs) were also evaluated to assess chromatin status of the host. Our results reveal morphological disruption of all studied subnuclear domains and severe reduction of viral progeny in PML-knockdown cells. ASFV promotes H3K9me3 and HP1β foci formation from early infection, followed by HP1α and HDAC2 nuclear enrichment, suggesting heterochromatinization of host genome. Finally, closeness between DNA damage response factors, disrupted PML-NBs, and virus-induced heterochromatic regions were identified. In sum, our results demonstrate that ASFV orchestrates spatio-temporal nuclear rearrangements, changing subnuclear domains, relocating Ataxia Telangiectasia Mutated Rad-3 related (ATR)-related factors and promoting heterochromatinization, probably controlling transcription, repressing host gene expression, and favouring viral replication.
Keywords: African swine fever virus; Cajal bodies; heterochromatin protein 1; histone H3 methylation modifications; histone deacetylases; nuclear speckles; promyelocytic leukaemia nuclear bodies.
Figures





Similar articles
-
Early intranuclear replication of African swine fever virus genome modifies the landscape of the host cell nucleus.Virus Res. 2015 Dec 2;210:1-7. doi: 10.1016/j.virusres.2015.07.006. Epub 2015 Jul 13. Virus Res. 2015. PMID: 26183880
-
Sodium phenylbutyrate abrogates African swine fever virus replication by disrupting the virus-induced hypoacetylation status of histone H3K9/K14.Virus Res. 2017 Oct 15;242:24-29. doi: 10.1016/j.virusres.2017.09.009. Epub 2017 Sep 13. Virus Res. 2017. PMID: 28916365
-
Host DNA damage response facilitates African swine fever virus infection.Vet Microbiol. 2013 Jul 26;165(1-2):140-7. doi: 10.1016/j.vetmic.2013.01.007. Epub 2013 Jan 23. Vet Microbiol. 2013. PMID: 23398667
-
Nuclear bodies and compartments: functional roles and cellular signalling in health and disease.Cell Signal. 2004 Oct;16(10):1085-104. doi: 10.1016/j.cellsig.2004.03.020. Cell Signal. 2004. PMID: 15240004 Review.
-
PML nuclear bodies and chromatin dynamics: catch me if you can!Nucleic Acids Res. 2020 Dec 2;48(21):11890-11912. doi: 10.1093/nar/gkaa828. Nucleic Acids Res. 2020. PMID: 33068409 Free PMC article. Review.
Cited by
-
Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC).Viruses. 2023 May 30;15(6):1283. doi: 10.3390/v15061283. Viruses. 2023. PMID: 37376583 Free PMC article.
-
Combinational Deletions of MGF360-9L and MGF505-7R Attenuated Highly Virulent African Swine Fever Virus and Conferred Protection against Homologous Challenge.J Virol. 2022 Jul 27;96(14):e0032922. doi: 10.1128/jvi.00329-22. Epub 2022 Jul 6. J Virol. 2022. PMID: 35867564 Free PMC article.
-
Inhibition of BET Family Proteins Suppresses African Swine Fever Virus Infection.Microbiol Spectr. 2022 Aug 31;10(4):e0241921. doi: 10.1128/spectrum.02419-21. Epub 2022 Jun 27. Microbiol Spectr. 2022. PMID: 35758684 Free PMC article.
-
Anti-viral role of toll like receptor 4 in hepatitis B virus infection: An in vitro study.World J Gastroenterol. 2016 Dec 21;22(47):10341-10352. doi: 10.3748/wjg.v22.i47.10341. World J Gastroenterol. 2016. PMID: 28058014 Free PMC article.
-
Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review.Front Immunol. 2021 Sep 6;12:715582. doi: 10.3389/fimmu.2021.715582. eCollection 2021. Front Immunol. 2021. PMID: 34552586 Free PMC article. Review.
References
-
- European Commission African Swine Fever. EFSA Panel on Animal Health and Welfare (AHAW) EFSA J. 2015;13 doi: 10.2903/j.efsa.2015.4163. - DOI
-
- King A.M.Q., Lefkowitz E., Adams M.J., Carstens E.B. Ninth report of the International Committee on Taxonomy of Viruses. In: King A.M.Q., editor. Virus Taxonomy. Elsevier; London, UK: 2011. pp. 153–162.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

