Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel
- PMID: 26389953
- PMCID: PMC4591660
- DOI: 10.3390/toxins7093671
Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel
Abstract
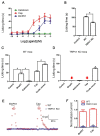
The intense pain induced by scorpion sting is a frequent clinical manifestation. To date, there is no established protocol with significant efficacy to alleviate the pain induced by scorpion envenomation. One of the important reasons is that, little information on pain-inducing compound from scorpion venoms is available. Here, a pain-inducing peptide (BmP01) has been identified and characterized from the venoms of scorpion (Mesobuthus martensii). In an animal model, intraplantar injection of BmP01 in mouse hind paw showed significant acute pain in wild type (WT) mice but not in TRPV1 knock-out (TRPV1 KO) mice during 30 min recording. BmP01 evoked currents in WT dorsal root ganglion (DRG) neurons but had no effect on DRG neurons of TRPV1 KO mice. Furthermore, OPEN ACCESS Toxins 2015, 7 3672 BmP01 evoked currents on TRPV1-expressed HEK293T cells, but not on HEK293T cells without TRPV1. These results suggest that (1) BmP01 is one of the pain-inducing agents in scorpion venoms; and (2) BmP01 induces pain by acting on TRPV1. To our knowledge, this is the first report about a scorpion toxin that produces pain by targeting TRPV1. Identification of a pain-inducing compound may facilitate treating pain induced by scorpion envenomation.
Keywords: BmP01; Kv channels; TRPV1; pain; peptide toxin; scorpion Mesobuthus martensii.
Figures




References
-
- Huang J., Yang Y., Dib-Hajj S.D., van Es M., Zhao P., Salomon J., Drenth J.P., Waxman S.G. Depolarized inactivation overcomes impaired activation to produce DRG neuron hyperexcitability in a NaV1.7 mutation in a patient with distal limb pain. J. Neurosci. 2014;34:12328–12340. doi: 10.1523/JNEUROSCI.2773-14.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

