In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles
- PMID: 26390044
- PMCID: PMC4648695
- DOI: 10.1039/c5cs00541h
In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles
Abstract
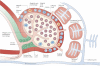
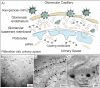
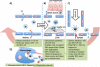
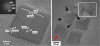
Iron oxide nanoparticles (IONPs) have been extensively used during the last two decades, either as effective bio-imaging contrast agents or as carriers of biomolecules such as drugs, nucleic acids and peptides for controlled delivery to specific organs and tissues. Most of these novel applications require elaborate tuning of the physiochemical and surface properties of the IONPs. As new IONPs designs are envisioned, synergistic consideration of the body's innate biological barriers against the administered nanoparticles and the short and long-term side effects of the IONPs become even more essential. There are several important criteria (e.g. size and size-distribution, charge, coating molecules, and plasma protein adsorption) that can be effectively tuned to control the in vivo pharmacokinetics and biodistribution of the IONPs. This paper reviews these crucial parameters, in light of biological barriers in the body, and the latest IONPs design strategies used to overcome them. A careful review of the long-term biodistribution and side effects of the IONPs in relation to nanoparticle design is also given. While the discussions presented in this review are specific to IONPs, some of the information can be readily applied to other nanoparticle systems, such as gold, silver, silica, calcium phosphates and various polymers.
Figures








References
-
- Rosen JE, Chan L, Shieh DB, Gu FX. Nanomedicine-Nanotechnology Biology and Medicine. 2012;8:275–290. - PubMed
-
- Arami H, Stephen Z, Veiseh O, Zhang M. Advances in Polymer Science. 2011;243:163–184.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

