Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI
- PMID: 26390357
- PMCID: PMC4715675
- DOI: 10.1002/mrm.25994
Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI
Abstract
Purpose: To demonstrate the feasibility of using ultrashort echo-time MRI to quantify T1 changes in cortical bone due to heating.
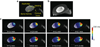
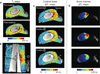
Methods: Variable flip-angle T1 mapping combined with 3D ultrashort echo-time imaging was used to measure T1 in cortical bone. A calibration experiment was performed to detect T1 changes with temperature in ex vivo cortical bone samples from a bovine femur. Ultrasound heating experiments were performed using an interstitial applicator in ex vivo bovine femur specimens, and heat-induced T1 changes were quantified.
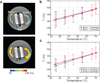
Results: The calibration experiment demonstrated that T1 increases with temperature in cortical bone. We observed a linear relationship between temperature and T1 with a linear coefficient between 0.67 and 0.84 ms/°C over a range of 25-70°C. The ultrasound heating experiments showed increased T1 changes in the heated regions, and the relationship between the temperature changes and T1 changes was similar to that of the calibration.
Conclusion: We demonstrated a temperature dependence of T1 in ex vivo cortical bone using a variable flip-angle ultrashort echo-time T1 mapping method.
Keywords: MR temperature mapping; T1 mapping; UTE imaging; cortical bone.
© 2015 Wiley Periodicals, Inc.
Figures


References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
-
- Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. 2004;2:205–213. - PubMed
-
- Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Atwell TD, Farrell MA, Welch TJ, Maus TP. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15. - PubMed
-
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—preliminary clinical experience. Ann Oncol. 2007;18:163–167. - PubMed
-
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16:140–146. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

