Metabolome and proteome changes with aging in Caenorhabditis elegans
- PMID: 26390854
- PMCID: PMC5473633
- DOI: 10.1016/j.exger.2015.09.013
Metabolome and proteome changes with aging in Caenorhabditis elegans
Abstract
To expand the understanding of aging in the model organism Caenorhabditis elegans, global quantification of metabolite and protein levels in young and aged nematodes was performed using mass spectrometry. With age, there was a decreased abundance of proteins functioning in transcription termination, mRNA degradation, mRNA stability, protein synthesis, and proteasomal function. Furthermore, there was altered S-adenosyl methionine metabolism as well as a decreased abundance of the S-adenosyl methionine synthetase (SAMS-1) protein. Other aging-related changes included alterations in free fatty acid levels and composition, decreased levels of ribosomal proteins, decreased levels of NADP-dependent isocitrate dehydrogenase (IDH1), a shift in the cellular redox state, an increase in sorbitol content, alterations in free amino acid levels, and indications of altered muscle function and sarcoplasmic reticulum Ca(2+) homeostasis. There were also decreases in pyrimidine and purine metabolite levels, most markedly nitrogenous bases. Supplementing the culture medium with cytidine (a pyrimidine nucleoside) or hypoxanthine (a purine base) increased lifespan slightly, suggesting that aging-induced alterations in ribonucleotide metabolism affect lifespan. An age-related increase in body size, lipotoxicity from ectopic yolk lipoprotein accumulation, a decline in NAD(+) levels, and mitochondrial electron transport chain dysfunction may explain many of these changes. In addition, dietary restriction in aged worms resulting from sarcopenia of the pharyngeal pump likely decreases the abundance of SAMS-1, possibly leading to decreased phosphatidylcholine levels, larger lipid droplets, and ER and mitochondrial stress. The complementary use of proteomics and metabolomics yielded unique insights into the molecular processes altered with age in C. elegans.
Keywords: Aging; Ascorbate; C. elegans; Caenorhabditis; Lifespan; Metabolome; Metabolomics; Methionine; Methylation; Methyltransferase; Nitrogenous bases; Proteome; Proteomics; Purine; Pyrimidine; S-adenosyl methionine; SAMS-1; SERCA; Sorbitol.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


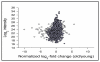
References
-
- Ackerman D, Gems D. The mystery of C. elegans aging: an emerging role for fat. Distant parallels between C. elegans aging and metabolic syndrome? Bioessays. 2012;34:466–471. - PubMed
-
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. - PubMed
-
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. - PubMed
-
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

