Neoadjuvant trials in early breast cancer: pathological response at surgery and correlation to longer term outcomes - what does it all mean?
- PMID: 26391216
- PMCID: PMC4578850
- DOI: 10.1186/s12916-015-0472-7
Neoadjuvant trials in early breast cancer: pathological response at surgery and correlation to longer term outcomes - what does it all mean?
Abstract
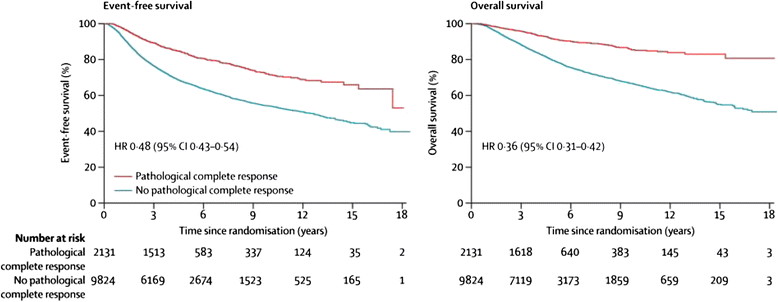
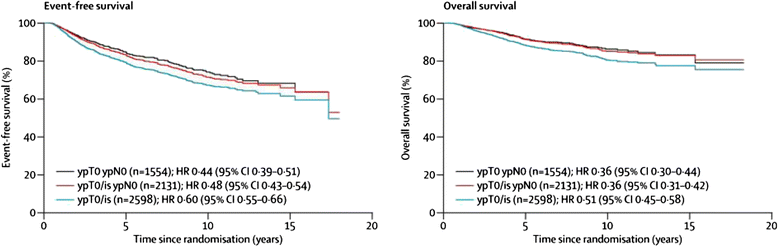
Background: Neoadjuvant breast cancer trials are important for speeding up the introduction of new treatments for patients with early breast cancer and for the highly productive translational research which they facilitate. Meta-analysis of trial data shows clear correlation between pathological response at surgery after neoadjuvant chemotherapy and longer-term outcomes at an individual patient level. However, this does not appear to be present on individual trial level analysis, when correlating improved outcome for the investigational arm for the primary endpoint (pathological response) with longer-term outcomes.
Discussion: The correlation between pathological response and longer-term outcomes in trials is dependent on many factors. These include definitions of pathological response, both complete and partial; assessment methods for pathological response at surgery; subtype and prognosis of breast cancer at diagnosis; number of patients recruited; adjuvant treatments; the mechanism of action of the investigational drug; the length of follow-up at the time of reporting; the definitions used in longer-term outcomes analysis; clonal heterogeneity; and new adaptive trial designs with additional neo/adjuvant treatments. Future developments of neoadjuvant breast cancer trials are discussed. With so many factors influencing the correlation of longer-term outcomes for trial-level data, we conclude that the main focus of neoadjuvant trials should remain the primary endpoint of pathological response. Neoadjuvant breast cancer trials are very important investigational studies that will continue to increase our understanding of the disease and offer the potential of more rapid introduction of new treatments for women with high-risk early breast cancer. In the future, we are likely to see both novel trial designs adopted in the neoadjuvant context and modifications of neo/adjuvant treatments for pathological non-responders within clinical trials. Both of these have the intention of improving longer-term outcomes for patients who do not have a good pathological response to first-line neoadjuvant treatment. If successful, these developments are likely to reduce further any positive correlation between pathological response and longer-term outcomes.
Figures


References
-
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. - PubMed
-
- Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Lluch A, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–81. doi: 10.1200/JCO.2008.19.2567. - DOI - PubMed
-
- Earl HM, Hiller L, Dunn JA, Blenkinsop C, Grybowicz L, Vallier AL, et al. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer (ARTemis): an open-label randomised phase 3 trial. Lancet Oncol. 2015;16:656–66. doi: 10.1016/S1470-2045(15)70137-3. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

