Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential
- PMID: 26391588
- PMCID: PMC4585749
- DOI: 10.1038/srep14218
Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential
Abstract
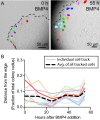
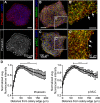
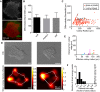
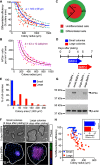
In order to understand the mechanisms that guide cell fate decisions during early human development, we closely examined the differentiation process in adherent colonies of human embryonic stem cells (hESCs). Live imaging of the differentiation process reveals that cells on the outer edge of the undifferentiated colony begin to differentiate first and remain on the perimeter of the colony to eventually form a band of differentiation. Strikingly, this band is of constant width in all colonies, independent of their size. Cells at the edge of undifferentiated colonies show distinct actin organization, greater myosin activity and stronger traction forces compared to cells in the interior of the colony. Increasing the number of cells at the edge of colonies by plating small colonies can increase differentiation efficiency. Our results suggest that human developmental decisions are influenced by cellular environments and can be dictated by colony geometry of hESCs.
Conflict of interest statement
There is a potential competing interest. Dr. Horsley has been funded by and received compensation in a consulting role for Unilever and Schick.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

