Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis
- PMID: 26391655
- PMCID: PMC4824610
- DOI: 10.1080/15548627.2015.1096485
Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis
Abstract
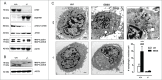
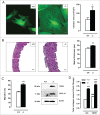
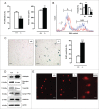
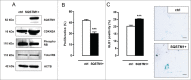
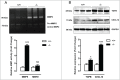
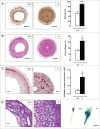
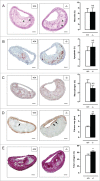
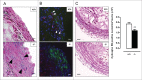
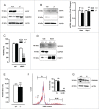
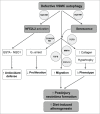
Autophagy is triggered in vascular smooth muscle cells (VSMCs) of diseased arterial vessels. However, the role of VSMC autophagy in cardiovascular disease is poorly understood. Therefore, we investigated the effect of defective autophagy on VSMC survival and phenotype and its significance in the development of postinjury neointima formation and atherosclerosis. Tissue-specific deletion of the essential autophagy gene Atg7 in murine VSMCs (atg7-/- VSMCs) caused accumulation of SQSTM1/p62 and accelerated the development of stress-induced premature senescence as shown by cellular and nuclear hypertrophy, CDKN2A-RB-mediated G1 proliferative arrest and senescence-associated GLB1 activity. Transfection of SQSTM1-encoding plasmid DNA in Atg7+/+ VSMCs induced similar features, suggesting that accumulation of SQSTM1 promotes VSMC senescence. Interestingly, atg7-/- VSMCs were resistant to oxidative stress-induced cell death as compared to controls. This effect was attributed to nuclear translocation of the transcription factor NFE2L2 resulting in upregulation of several antioxidative enzymes. In vivo, defective VSMC autophagy led to upregulation of MMP9, TGFB and CXCL12 and promoted postinjury neointima formation and diet-induced atherogenesis. Lesions of VSMC-specific atg7 knockout mice were characterized by increased total collagen deposition, nuclear hypertrophy, CDKN2A upregulation, RB hypophosphorylation, and GLB1 activity, all features typical of cellular senescence. To conclude, autophagy is crucial for VSMC function, phenotype, and survival. Defective autophagy in VSMCs accelerates senescence and promotes ligation-induced neointima formation and diet-induced atherogenesis, implying that autophagy inhibition as therapeutic strategy in the treatment of neointimal stenosis and atherosclerosis would be unfavorable. Conversely, stimulation of autophagy could be a valuable new strategy in the treatment of arterial disease.
Keywords: atherosclerosis; autophagy; neointima formation; senescence; sequestosome 1/p62; vascular smooth muscle cells.
Figures


References
-
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014; 24:24-41; PMID:24366339; http://dx.doi.org/10.1038/cr.2013.168 - DOI - PMC - PubMed
-
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011; 147:728-41; PMID:22078875; http://dx.doi.org/10.1016/j.cell.2011.10.026 - DOI - PubMed
-
- Martinet W, De Bie M, Schrijvers DM, De Meyer GRY, Herman AG, Kockx MM. 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2004; 24:2296-301; PMID:15458974; http://dx.doi.org/10.1161/01.ATV.0000146266.65820.a1 - DOI - PubMed
-
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-α regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol 2006; 84:448-54; PMID:16942488; http://dx.doi.org/10.1111/j.1440-1711.2006.01454.x - DOI - PubMed
-
- Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J 2008; 410:525-34; PMID:18052926; http://dx.doi.org/10.1042/BJ20071063 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
