Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually
- PMID: 26392012
- PMCID: PMC4609623
- DOI: 10.1016/j.vaccine.2015.08.086
Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually
Abstract
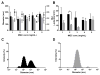
Influenza is a vaccine-preventable contagious respiratory illness caused by influenza (flu) viruses which can lead to hospitalization and sometimes even death. Current flu vaccines delivered intramuscularly (IM) or intradermally (ID) are less effective at eliciting protective mucosal immune responses and vaccines delivered intranasally (IN) possess potential safety concerns. Sublingual (SL) vaccination is a promising alternative route for vaccine delivery which has been indicated as safe and effective at inducing protective immune responses in both systemic and mucosal compartments. We evaluated the efficacy of methylglycol chitosan (MGC) and a synthetic toll-like receptor 4 agonist (CRX-601), alone or in combination, for improving systemic and mucosal immune responses to a monovalent detergent-split flu virus vaccine delivered SL. SL vaccination of mice with split-flu vaccine formulated with either MGC or CRX-601 resulted in specific serum IgG and mucosal IgA titers that were significantly greater than titers from non-adjuvanted vaccination and equivalent to or greater than titers in mice vaccinated IM. Our results demonstrate that SL vaccination utilizing MGC or CRX-601 as adjuvants is a viable alternative route of vaccination for flu which can elicit systemic immune responses equivalent to or greater than IM vaccination with the added benefit of stimulating a robust specific mucosal immune response.
Keywords: CRX-601; Chitosan; Influenza; Mucosal vaccination; Sublingual; TLR-4.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors were employees of GSK-Vaccines during performance of the work reported herein.
Figures





References
-
- Couch RB, Kasel JA, Glezen WP, Cate TR, Six HR, Taber LH, et al. Influenza: its control in persons and populations. J Infect Dis. 1986;153:431–40. - PubMed
-
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. - PMC - PubMed
-
- Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–15. - PubMed
-
- Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

