Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial
- PMID: 26392097
- PMCID: PMC4980566
- DOI: 10.1200/JCO.2015.61.8413
Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial
Abstract
Purpose: Significant improvement in survival outcomes has been established with the addition of trastuzumab to adjuvant chemotherapy for human epidermal growth factor receptor 2 (HER2) -positive early breast cancer treatment. However, trastuzumab may increase the risk of cardiac toxicity, and long-term evaluation of its incidence and risk factors are warranted.
Methods: NCCTG (Alliance) N9831 trial compared adjuvant doxorubicin and cyclophosphamide (AC) followed by either weekly paclitaxel (arm A); paclitaxel then trastuzumab (arm B); or paclitaxel plus trastuzumab followed by trastuzumab alone (arm C) in patients with HER2-positive breast cancer. Cumulative incidence of cardiac events (CE) and left ventricular ejection fraction (LVEF) were evaluated in 1,944 women who proceeded to post-AC therapy. Risk factors for trastuzumab-induced cardiac toxicity were identified by Cox regression models.
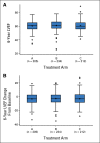
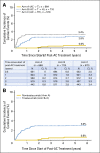
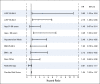
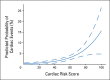
Results: The 6-year cumulative incidence of CE was 0.6% in arm A, 2.8% in arm B, and 3.4% in arm C. At a median follow-up of 9.2 years, only two additional CHF diagnoses (of 1,046 patients) occurred beyond our previously reported follow-up time of 3.75 years. LVEF recovered in the majority of the patients who developed CHF. There were two cardiac deaths in arm A and one each in arms B and C. Age of 60 years or older, registration LVEF less than 65%, and use of antihypertensive medications were associated with an increased risk of CE in arms B and C.
Conclusion: The cumulative incidence of CE at 6 years was slightly higher with the addition of trastuzumab; however, the late development of CE is infrequent. Trastuzumab (in the context of anthracycline- and taxane-based therapy) continues to have a favorable benefit-risk ratio.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6,556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

