Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer
- PMID: 26392102
- PMCID: PMC4669588
- DOI: 10.1200/JCO.2015.63.2471
Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer
Abstract
Purpose: To evaluate dabrafenib, a selective BRAF inhibitor, combined with trametinib, a selective MEK inhibitor, in patients with BRAF V600-mutant metastatic colorectal cancer (mCRC).
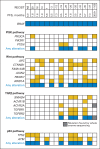
Patients and methods: A total of 43 patients with BRAF V600-mutant mCRC were treated with dabrafenib (150 mg twice daily) plus trametinib (2 mg daily), 17 of whom were enrolled onto a pharmacodynamic cohort undergoing mandatory biopsies before and during treatment. Archival tissues were analyzed for microsatellite instability, PTEN status, and 487-gene sequencing. Patient-derived xenografts were established from core biopsy samples.
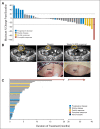
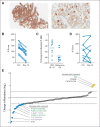
Results: Of 43 patients, five (12%) achieved a partial response or better, including one (2%) complete response, with duration of response > 36 months; 24 patients (56%) achieved stable disease as best confirmed response. Ten patients (23%) remained in the study > 6 months. All nine evaluable during-treatment biopsies had reduced levels of phosphorylated ERK relative to pretreatment biopsies (average decrease ± standard deviation, 47% ± 24%). Mutational analysis revealed that the patient achieving a complete response and two of three evaluable patients achieving a partial response had PIK3CA mutations. Neither PTEN loss nor microsatellite instability correlated with efficacy. Responses to dabrafenib plus trametinib were comparable in patient-derived xenograft-bearing mice and the biopsied lesions from each corresponding patient.
Conclusion: The combination of dabrafenib plus trametinib has activity in a subset of patients with BRAF V600-mutant mCRC. Mitogen-activated protein kinase signaling was inhibited in all patients evaluated, but to a lesser degree than observed in BRAF-mutant melanoma with dabrafenib alone. PIK3CA mutations were identified in responding patients and thus do not preclude response to this regimen. Additional studies targeting the mitogen-activated protein kinase pathway in this disease are warranted.
Trial registration: ClinicalTrials.gov NCT01072175.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures


Comment in
-
Hitting the Target in BRAF-Mutant Colorectal Cancer.J Clin Oncol. 2015 Dec 1;33(34):3990-2. doi: 10.1200/JCO.2015.63.7793. Epub 2015 Oct 12. J Clin Oncol. 2015. PMID: 26460302 No abstract available.
References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084. - PubMed
-
- Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. - PubMed
-
- Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675–2682. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- R01CA172670/CA/NCI NIH HHS/United States
- K08CA166510/CA/NCI NIH HHS/United States
- R01CA184843/CA/NCI NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- R01 CA184843/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- R01 CA172670/CA/NCI NIH HHS/United States
- K08 CA175153/CA/NCI NIH HHS/United States
- P50CA127003/CA/NCI NIH HHS/United States
- K08CA175143/CA/NCI NIH HHS/United States
- UL1 RR024148/RR/NCRR NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- K08 CA166510/CA/NCI NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

