Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage
- PMID: 26392223
- PMCID: PMC4612094
- DOI: 10.1084/jem.20150318
Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage
Abstract
Disruption of the intestinal epithelial barrier allows bacterial translocation and predisposes to destructive inflammation. To ensure proper barrier composition, crypt-residing stem cells continuously proliferate and replenish all intestinal epithelial cells within days. As a consequence of this high mitotic activity, mucosal surfaces are frequently targeted by anticancer therapies, leading to dose-limiting side effects. The cellular mechanisms that control tissue protection and mucosal healing in response to intestinal damage remain poorly understood. Type 3 innate lymphoid cells (ILC3s) are regulators of homeostasis and tissue responses to infection at mucosal surfaces. We now demonstrate that ILC3s are required for epithelial activation and proliferation in response to small intestinal tissue damage induced by the chemotherapeutic agent methotrexate. Multiple subsets of ILC3s are activated after intestinal tissue damage, and in the absence of ILC3s, epithelial activation is lost, correlating with increased pathology and severe damage to the intestinal crypts. Using ILC3-deficient Lgr5 reporter mice, we show that maintenance of intestinal stem cells after damage is severely impaired in the absence of ILC3s or the ILC3 signature cytokine IL-22. These data unveil a novel function of ILC3s in limiting tissue damage by preserving tissue-specific stem cells.
© 2015 Aparicio-Domingo et al.
Figures
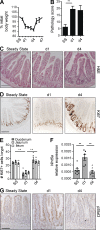

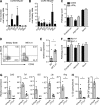


Comment in
-
ILC3s protect intestinal stem cells from chemotherapy.J Exp Med. 2015 Oct 19;212(11):1756. doi: 10.1084/jem.21211insight3. J Exp Med. 2015. PMID: 26482141 Free PMC article. No abstract available.
References
-
- Anderson C.A., Boucher G., Lees C.W., Franke A., D’Amato M., Taylor K.D., Lee J.C., Goyette P., Imielinski M., Latiano A., et al. . 2011. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43:246–252. 10.1038/ng.764 - DOI - PMC - PubMed
-
- Bollrath J., Phesse T.J., von Burstin V.A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., et al. . 2009. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 15:91–102. 10.1016/j.ccr.2009.01.002 - DOI - PubMed
-
- de Koning B.A., van Dieren J.M., Lindenbergh-Kortleve D.J., van der Sluis M., Matsumoto T., Yamaguchi K., Einerhand A.W., Samsom J.N., Pieters R., and Nieuwenhuis E.E.. 2006. Contributions of mucosal immune cells to methotrexate-induced mucositis. Int. Immunol. 18:941–949. 10.1093/intimm/dxl030 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

