Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection
- PMID: 26392224
- PMCID: PMC4612096
- DOI: 10.1084/jem.20150632
Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection
Abstract
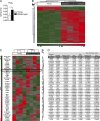
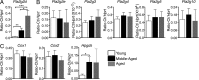
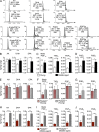
Oxidative stress and chronic low-grade inflammation in the lungs are associated with aging and may contribute to age-related immune dysfunction. To maintain lung homeostasis, chronic inflammation is countered by enhanced expression of proresolving/antiinflammatory factors. Here, we show that age-dependent increases of one such factor in the lungs, a phospholipase A2 (PLA2) group IID (PLA2G2D) with antiinflammatory properties, contributed to worse outcomes in mice infected with severe acute respiratory syndrome-coronavirus (SARS-CoV). Strikingly, infection of mice lacking PLA2G2D expression (Pla2g2d(-/-) mice) converted a uniformly lethal infection to a nonlethal one (>80% survival), subsequent to development of enhanced respiratory DC migration to the draining lymph nodes, augmented antivirus T cell responses, and diminished lung damage. We also observed similar effects in influenza A virus-infected middle-aged Pla2g2d(-/-) mice. Furthermore, oxidative stress, probably via lipid peroxidation, was found to induce PLA2G2D expression in mice and in human monocyte-derived macrophages. Thus, our results suggest that directed inhibition of a single inducible phospholipase, PLA2G2D, in the lungs of older patients with severe respiratory infections is potentially an attractive therapeutic intervention to restore immune function.
© 2015 Vijay et al.
Figures





Comment in
-
A phospholipase linkAGE to SARS susceptibility.J Exp Med. 2015 Oct 19;212(11):1755. doi: 10.1084/jem.21211insight2. J Exp Med. 2015. PMID: 26482140 Free PMC article. No abstract available.
References
-
- Angeli V., Faveeuw C., Roye O., Fontaine J., Teissier E., Capron A., Wolowczuk I., Capron M., and Trottein F.. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135–1147. 10.1084/jem.193.10.1135 - DOI - PMC - PubMed
-
- Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J.N., Petasis N.A., Blumberg R.S., and Serhan C.N.. 2005. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA. 102:7671–7676. 10.1073/pnas.0409271102 - DOI - PMC - PubMed
-
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., et al. . 2013. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 13:752–761. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

